Iron(III) chloride describes the inorganic compounds with the formula FeCl3(H2O)x. Also called ferric chloride, these compounds are some of the most important and commonplace compounds of iron. They are available both in anhydrous and in hydrated forms which are both hygroscopic. They feature iron in its +3 oxidation state. The anhydrous derivative is a Lewis acid, while all forms are mild oxidizing agents. It is used as a water cleaner and as an etchant for metals.
Image: Iron(III) chloride anhydrate
Image: Хлорид железа
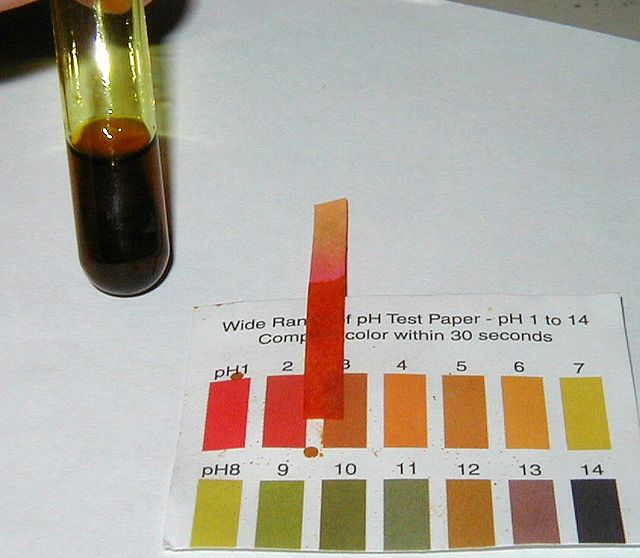
A brown, acidic solution of iron(III) chloride.
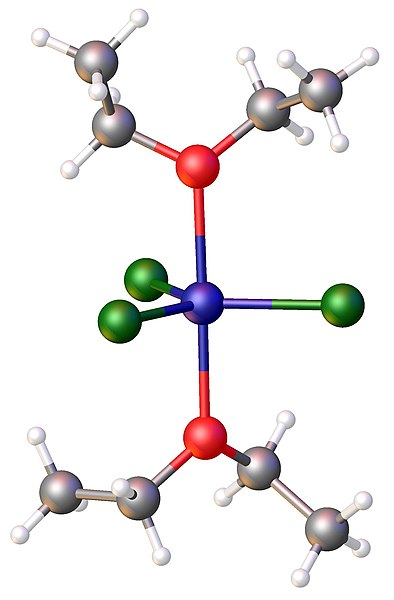
Structure of FeCl3(diethylether)2. Color code: Cl=green,Fe = blue, O = red.
Hygroscopy is the phenomenon of attracting and holding water molecules via either absorption or adsorption from the surrounding environment, which is usually at normal or room temperature. If water molecules become suspended among the substance's molecules, adsorbing substances can become physically changed, e.g. changing in volume, boiling point, viscosity or some other physical characteristic or property of the substance. For example, a finely dispersed hygroscopic powder, such as a salt, may become clumpy over time due to collection of moisture from the surrounding environment.
Air plant (Tillandsia bulbosa)
The aquatic file snake (A. granulatus) with hygroscopic skin, shown out of water
An orb-weaver spider (Larinioides cornutus) with hygroscopic coated capture threads
Waxy monkey tree frog (Phyllomedusa sauvagii)