Redox is a type of chemical reaction in which the oxidation states of a reactant change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a decrease in the oxidation state.
Oxides, such as iron(III) oxide or rust, which consists of hydrated iron(III) oxides Fe2O3·nH2O and iron(III) oxide-hydroxide (FeO(OH), Fe(OH)3), form when oxygen combines with other elements.
Iron rusting in pyrite cubes
Enzymatic browning is an example of a redox reaction that takes place in most fruits and vegetables.
Blast furnaces of Třinec Iron and Steel Works, Czech Republic
A chemical reaction is a process that leads to the chemical transformation of one set of chemical substances to another. When chemical reactions occur, the atoms are rearranged and the reaction is accompanied by an energy change as new products are generated. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking of chemical bonds between atoms, with no change to the nuclei, and can often be described by a chemical equation. Nuclear chemistry is a sub-discipline of chemistry that involves the chemical reactions of unstable and radioactive elements where both electronic and nuclear changes can occur.
A thermite reaction using iron(III) oxide. The sparks flying outwards are globules of molten iron trailing smoke in their wake.
Antoine Lavoisier developed the theory of combustion as a chemical reaction with oxygen.
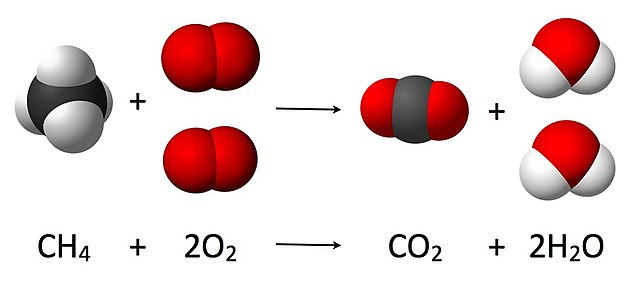
As seen from the equation CH4 + 2O2 → CO2 + 2 H2O, a coefficient of 2 must be placed before the oxygen gas on the reactants side and before the water on the products side in order for, as per the law of conservation of mass, the quantity of each element does not change during the reaction.
Sodium chloride is formed through the redox reaction of sodium metal and chlorine gas