Lorenzo Romano Amedeo Carlo Avogadro, Count of Quaregna and Cerreto (, also, Italian: [ameˈdɛːo avoˈɡaːdro]; 9 August 1776 – 9 July 1856) was an Italian scientist, most noted for his contribution to molecular theory now known as Avogadro's law, which states that equal volumes of gases under the same conditions of temperature and pressure will contain equal numbers of molecules. In tribute to him, the ratio of the number of elementary entities (atoms, molecules, ions or other particles) in a substance to its amount of substance (the latter having the unit mole), 6.02214076×1023 mol−1, is known as the Avogadro constant. This constant is denoted NA, and is one of the seven defining constants of the SI.
Essay on the mathematical theory of the distribution of electricity on the surface of conducting bodies, 1844
Mémoire
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and biochemistry, the distinction from ions is dropped and molecule is often used when referring to polyatomic ions.
Atomic force microscopy (AFM) image of a PTCDA molecule, in which the five six-carbon rings are visible.
A scanning tunneling microscopy image of pentacene molecules, which consist of linear chains of five carbon rings.
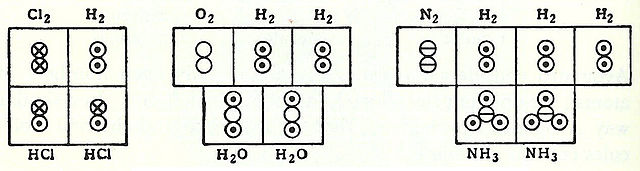
Marc Antoine Auguste Gaudin's volume diagrams of molecules in the gas phase (1833)
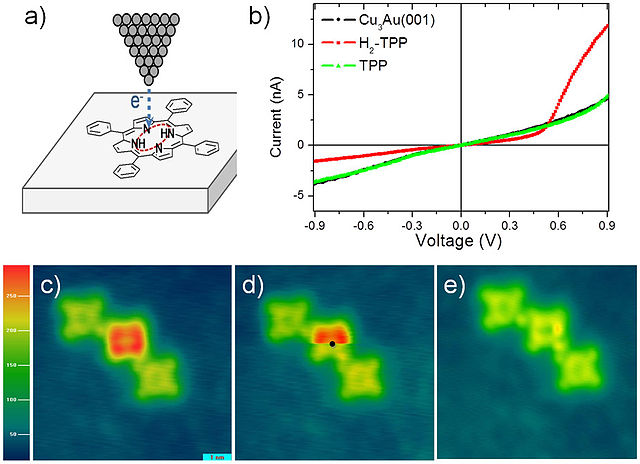
Hydrogen can be removed from individual H2TPP molecules by applying excess voltage to the tip of a scanning tunneling microscope (STM, a); this removal alters the current-voltage (I-V) curves of TPP molecules, measured using the same STM tip, from diode like (red curve in b) to resistor like (green curve). Image (c) shows a row of TPP, H2TPP and TPP molecules. While scanning image (d), excess voltage was applied to H2TPP at the black dot, which instantly removed hydrogen, as shown in the bottom part of (d) and in the rescan image (e). Such manipulations can be used in