Bacillus subtilis, known also as the hay bacillus or grass bacillus, is a gram-positive, catalase-positive bacterium, found in soil and the gastrointestinal tract of ruminants, humans and marine sponges. As a member of the genus Bacillus, B. subtilis is rod-shaped, and can form a tough, protective endospore, allowing it to tolerate extreme environmental conditions. B. subtilis has historically been classified as an obligate aerobe, though evidence exists that it is a facultative anaerobe. B. subtilis is considered the best studied Gram-positive bacterium and a model organism to study bacterial chromosome replication and cell differentiation. It is one of the bacterial champions in secreted enzyme production and used on an industrial scale by biotechnology companies.
Bacillus subtilis
Sporulating B. subtilis.
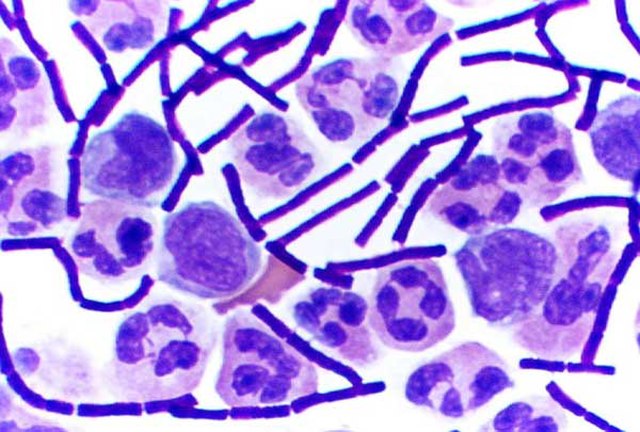
Gram-stained B. subtilis
Colonies of B. subtilis grown on a culture dish in a molecular biology laboratory.
In bacteriology, gram-positive bacteria are bacteria that give a positive result in the Gram stain test, which is traditionally used to quickly classify bacteria into two broad categories according to their type of cell wall.
Rod-shaped gram-positive Bacillus anthracis bacteria in a cerebrospinal fluid sample stand out from round white blood cells, which also accept the crystal violet stain.
Violet-stained gram-positive cocci and pink-stained gram-negative bacilli
Colonies of a gram-positive pathogen of the oral cavity, Actinomyces sp.