The CMYK color model is a subtractive color model, based on the CMY color model, used in color printing, and is also used to describe the printing process itself. The abbreviation CMYK refers to the four ink plates used: cyan, magenta, yellow, and key (black).
What appears as cerulean ( ) in the top image is actually a blend of cyan, magenta, yellow and black, as magnification under a microscope demonstrates.
A color photograph of the Teton Range.
Inspection CMYK colors of offset printing on a paper
Early representation of the three-color process (1902)
Subtractive color or subtractive color mixing predicts the spectral power distribution of light after it passes through successive layers of partially absorbing media. This idealized model is the essential principle of how dyes and pigments are used in color printing and photography, where the perception of color is elicited after white light passes through microscopic "stacks" of partially absorbing media allowing some wavelengths of light to reach the eye and not others, and also in painting, whether the colors are mixed or applied in successive layers.
An 1877 color photo by Louis Ducos du Hauron, a French pioneer of color photography. The overlapping subtractive yellow, cyan and red (magenta) image elements can be seen clearly along the edges of the image.
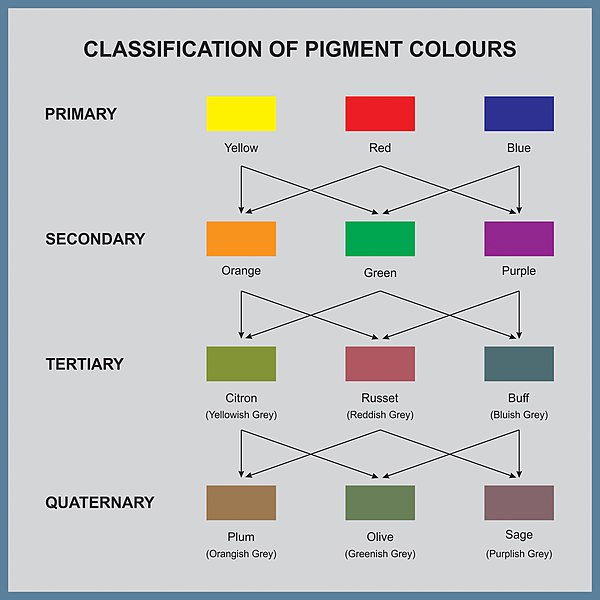
Classification of pigment colors
Cyan, magenta and yellow color filters