Carbon is a primary component of all known life on Earth, and represents approximately 45–50% of all dry biomass. Carbon compounds occur naturally in great abundance on Earth. Complex biological molecules consist of carbon atoms bonded with other elements, especially oxygen and hydrogen and frequently also nitrogen, phosphorus, and sulfur.
Plant cell wall (cellulose) and chloroplasts conduct photosynthesis in plant cells and other eukaryotic organisms.
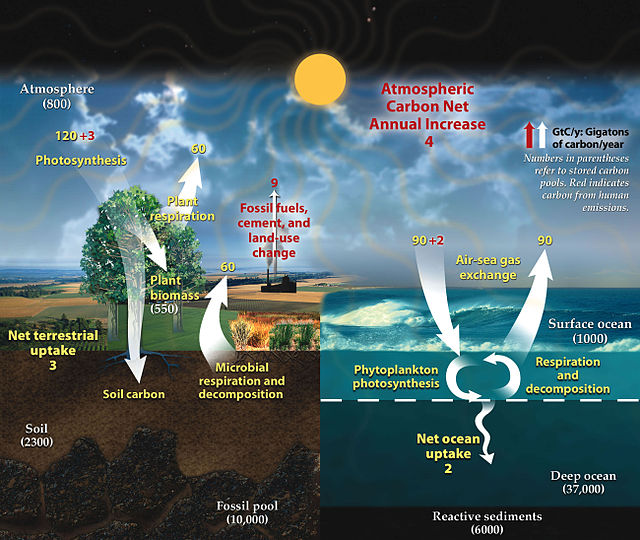
Fast carbon cycle showing the movement of carbon between land, atmosphere
Where carbon goes when water flows
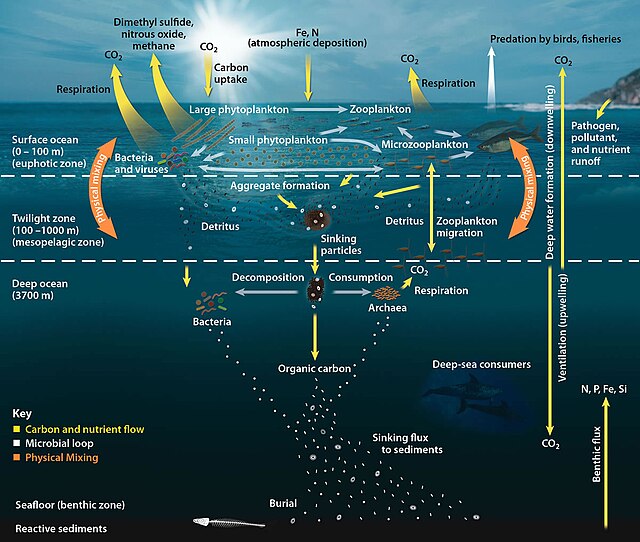
Oceanic carbon cycle
Carbon is a chemical element; it has symbol C and atomic number 6. It is nonmetallic and tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 electrons. It belongs to group 14 of the periodic table. Carbon makes up about 0.025 percent of Earth's crust. Three isotopes occur naturally, 12C and 13C being stable, while 14C is a radionuclide, decaying with a half-life of about 5,730 years. Carbon is one of the few elements known since antiquity.
Graphite (left) and diamond (right), two allotropes of carbon
A large sample of glassy carbon
Comet C/2014 Q2 (Lovejoy) surrounded by glowing carbon vapor
Graphite ore, shown with a penny for scale