Catechin is a flavan-3-ol, a type of secondary metabolite providing antioxidant roles in plants. It belongs to the subgroup of polyphenols called flavonoids.
Schematic representation of (−)-epicatechin metabolism in humans as a function of time post-oral intake. SREM: structurally related (−)-epicatechin metabolites. 5C-RFM: 5-carbon ring fission metabolites. 3/1C-RFM: 3- and 1-carbon-side chain ring fission metabolites. The structures of the most abundant (−)-epicatechin metabolites present in the systemic circulation and in urine are depicted.
Flavan-3-ols are a subgroup of flavonoids. They are derivatives of flavans that possess a 2-phenyl-3,4-dihydro-2H-chromen-3-ol skeleton. Flavan-3-ols are structurally diverse and include a range of compounds, such as catechin, epicatechin gallate, epigallocatechin, epigallocatechin gallate, proanthocyanidins, theaflavins, thearubigins. They play a part in plant defense and are present in the majority of plants.
Schematic representation of the flavan-3-ol (−)-epicatechin metabolism in humans as a function of time post-oral intake. SREM: structurally related (−)-epicatechin metabolites. 5C-RFM: 5-carbon ring fission metabolites. 3/1C-RFM: 3- and 1-carbon-side chain ring fission metabolites. The structures of the most abundant (−)-epicatechin metabolites present in the systemic circulation and in urine are depicted.
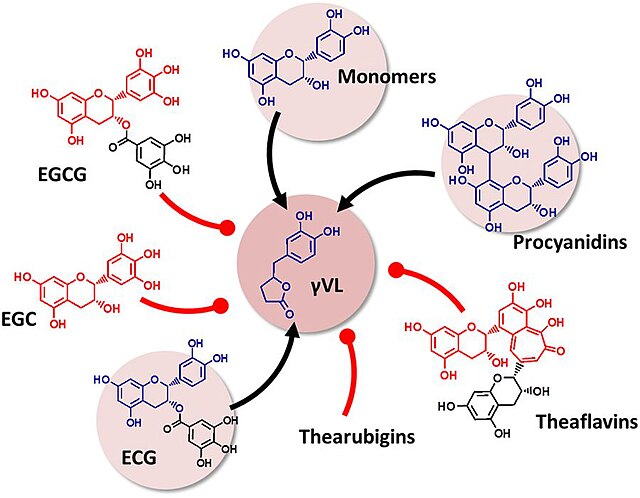
Flavan-3-ol precursors of the microbial metabolite 5-(3′/4′-dihydroxyphenyl)-γ-valerolactone (gVL). Only compounds with intact (epi)catechin moiety result in the formation of γVL by the intestinal microbiome. ECG, (−)-epicatechin-3-O-gallate; EGCG, Epigallocatechin gallate; EGC, Epigallocatechin.