Cathode rays or electron beams (e-beam) are streams of electrons observed in discharge tubes. If an evacuated glass tube is equipped with two electrodes and a voltage is applied, glass behind the positive electrode is observed to glow, due to electrons emitted from the cathode. They were first observed in 1859 by German physicist Julius Plücker and Johann Wilhelm Hittorf, and were named in 1876 by Eugen Goldstein Kathodenstrahlen, or cathode rays. In 1897, British physicist J. J. Thomson showed that cathode rays were composed of a previously unknown negatively charged particle, which was later named the electron. Cathode-ray tubes (CRTs) use a focused beam of electrons deflected by electric or magnetic fields to render an image on a screen.

A beam of cathode rays in a vacuum tube bent into a circle by a magnetic field generated by a Helmholtz coil. Cathode rays are normally invisible; in this demonstration Teltron tube, enough gas has been left in the tube for the gas atoms to luminesce when struck by the fast-moving electrons.
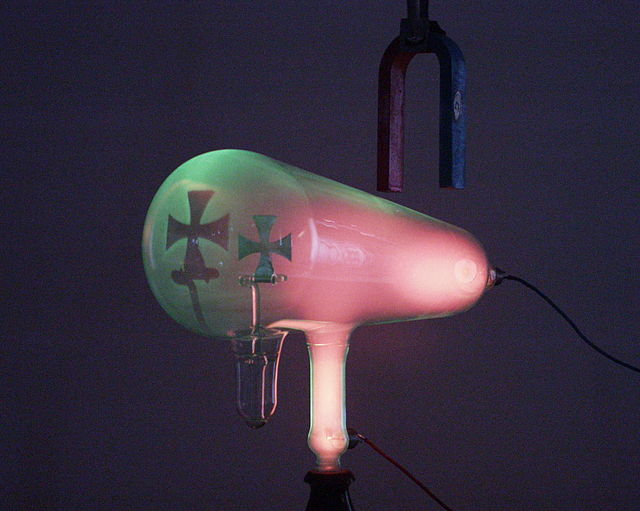
Crookes tube. The cathode (negative terminal) is on the right. The anode (positive terminal) is in the base of the tube at bottom.
Cathode rays travel from the cathode at the rear of the tube, striking the glass front, making it glow green by fluorescence. A metal cross in the tube casts a shadow, demonstrating that the rays travel in straight lines.
A magnet creates a horizontal magnetic field through the neck of the tube, bending the rays up, so the shadow of the cross is higher.
The electron is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family, and are generally thought to be elementary particles because they have no known components or substructure. The electron's mass is approximately 1/1836 that of the proton. Quantum mechanical properties of the electron include an intrinsic angular momentum (spin) of a half-integer value, expressed in units of the reduced Planck constant, ħ. Being fermions, no two electrons can occupy the same quantum state, per the Pauli exclusion principle. Like all elementary particles, electrons exhibit properties of both particles and waves: They can collide with other particles and can be diffracted like light. The wave properties of electrons are easier to observe with experiments than those of other particles like neutrons and protons because electrons have a lower mass and hence a longer de Broglie wavelength for a given energy.

A beam of electrons deflected by a magnetic field into a circle
J. J. Thomson
Robert Millikan
A lightning discharge consists primarily of a flow of electrons. The electric potential needed for lightning can be generated by a triboelectric effect.