Clinical trials are prospective biomedical or behavioral research studies on human participants designed to answer specific questions about biomedical or behavioral interventions, including new treatments and known interventions that warrant further study and comparison. Clinical trials generate data on dosage, safety and efficacy. They are conducted only after they have received health authority/ethics committee approval in the country where approval of the therapy is sought. These authorities are responsible for vetting the risk/benefit ratio of the trial—their approval does not mean the therapy is 'safe' or effective, only that the trial may be conducted.
A clinical trial participant receives an injection.
Edward Jenner vaccinating James Phipps, a boy of eight, on 14 May 1796. Jenner failed to use a control group.
Austin Bradford Hill was a pivotal figure in the modern development of clinical trials.
Timeline of various approval tracks and research phases in the US
The pharmaceutical industry is an industry in medicine that discovers, develops, produces, and markets pharmaceutical drugs for use as medications to be administered to patients, with the aim to cure and prevent diseases, or alleviate symptoms. Pharmaceutical companies may deal in generic or brand medications and medical devices. They are subject to a variety of laws and regulations that govern the patenting, testing, safety, efficacy using drug testing and marketing of drugs. The global pharmaceuticals market produced treatments worth $1,228.45 billion in 2020 and showed a compound annual growth rate (CAGR) of 1.8%.
A drug manufacturer inspection by the US Food and Drug Administration
Diethylbarbituric acid was the first marketed barbiturate. It was sold by Bayer under the trade name Veronal.
In 1937 over 100 people died after ingesting a solution of the antibacterial sulfanilamide formulated in the toxic solvent diethylene glycol.
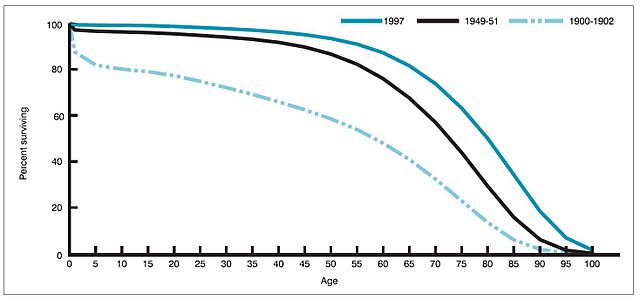
Percent surviving by age in 1900, 1950, and 1997