Cobalt-60 (60Co) is a synthetic radioactive isotope of cobalt with a half-life of 5.2714 years. It is produced artificially in nuclear reactors. Deliberate industrial production depends on neutron activation of bulk samples of the monoisotopic and mononuclidic cobalt isotope 59Co. Measurable quantities are also produced as a by-product of typical nuclear power plant operation and may be detected externally when leaks occur. In the latter case the incidentally produced 60Co is largely the result of multiple stages of neutron activation of iron isotopes in the reactor's steel structures via the creation of its 59Co precursor. The simplest case of the latter would result from the activation of 58Fe. 60Co undergoes beta decay to the stable isotope nickel-60. The activated cobalt nucleus emits two gamma rays with energies of 1.17 and 1.33 MeV, hence the overall equation of the nuclear reaction is: 5927Co + n → 6027Co → 6028Ni + e− + 2 γ

Security screening of cars at Super Bowl XLI using 60Co gamma-ray scanner (2007)
60Co needle implanted in tumors for radiotherapy, around 1955.
60Co teletherapy machine for cancer radiotherapy, early 1950s.
Brookhaven plant mutation experiment using 60Co source in the pipe, center.
Cobalt is a chemical element; it has symbol Co and atomic number 27. As with nickel, cobalt is found in the Earth's crust only in a chemically combined form, save for small deposits found in alloys of natural meteoric iron. The free element, produced by reductive smelting, is a hard, lustrous, silvery metal.
Cobalt
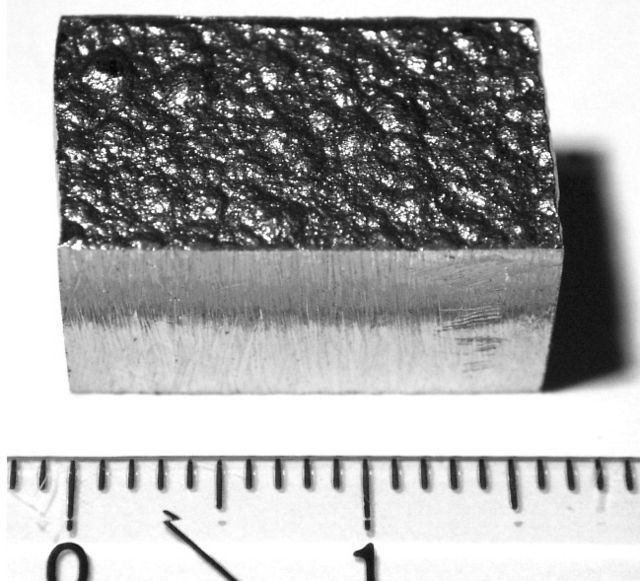
A block of electrolytically refined cobalt (99.9% purity) cut from a large plate
Cobalt(II) chloride hexahydrate
Early Chinese blue and white porcelain, manufactured c. 1335