Coccoliths are individual plates or scales of calcium carbonate formed by coccolithophores and cover the cell surface arranged in the form of a spherical shell, called a coccosphere.
Scanning electron micrograph of Coccolithus pelagicus, plated with coccoliths
Collapsed coccosphere of Pleurochrysis carterae
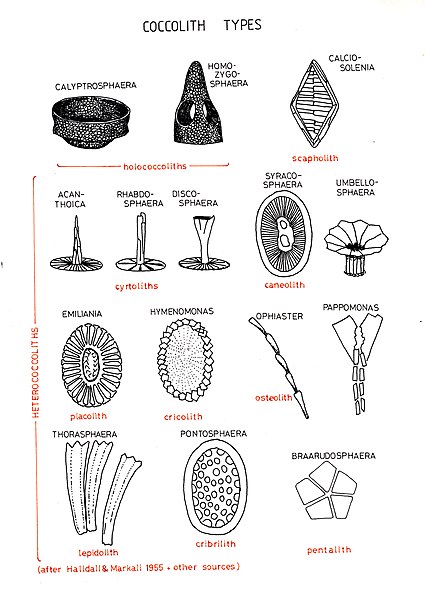
Types of coccoliths
Helicoliths of Helicosphaera carteri
Calcium carbonate is a chemical compound with the chemical formula CaCO3. It is a common substance found in rocks as the minerals calcite and aragonite, most notably in chalk and limestone, eggshells, gastropod shells, shellfish skeletons and pearls. Materials containing much calcium carbonate or resembling it are described as calcareous. Calcium carbonate is the active ingredient in agricultural lime and is produced when calcium ions in hard water react with carbonate ions to form limescale. It has medical use as a calcium supplement or as an antacid, but excessive consumption can be hazardous and cause hypercalcemia and digestive issues.
Calcium carbonate
Calcite is the most stable polymorph of calcium carbonate. It is transparent to opaque. A transparent variety called Iceland spar (shown here) was used to create polarized light in the 19th century.
Calcium carbonate chunks from clamshell
Surface precipitation of CaCO3 as tufa in Rubaksa, Ethiopia