Combustion, or burning, is a high-temperature exothermic redox chemical reaction between a fuel and an oxidant, usually atmospheric oxygen, that produces oxidized, often gaseous products, in a mixture termed as smoke. Combustion does not always result in fire, because a flame is only visible when substances undergoing combustion vaporize, but when it does, a flame is a characteristic indicator of the reaction. While activation energy must be supplied to initiate combustion, the heat from a flame may provide enough energy to make the reaction self-sustaining. The study of combustion is known as combustion science.
The flames caused as a result of a fuel undergoing combustion (burning)
Air pollution abatement equipment provides combustion control for industrial processes.
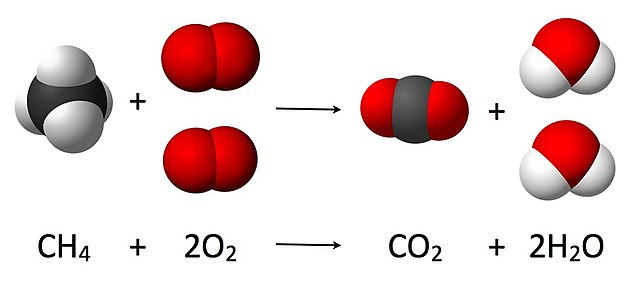
The combustion of methane, a hydrocarbon
Colourized gray-scale composite image of the individual frames from a video of a backlit fuel droplet burning in microgravity
In thermodynamics, an exothermic process is a thermodynamic process or reaction that releases energy from the system to its surroundings, usually in the form of heat, but also in a form of light, electricity, or sound. The term exothermic was first coined by 19th-century French chemist Marcellin Berthelot.
Explosions are some of the most violent exothermic reactions.
An exothermic thermite reaction using iron(III) oxide. The sparks flying outwards are globules of molten iron trailing smoke in their wake.