In physics and chemistry, the law of conservation of mass or principle of mass conservation states that for any system closed to all transfers of matter and energy, the mass of the system must remain constant over time, as the system's mass cannot change, so the quantity can neither be added nor be removed. Therefore, the quantity of mass is conserved over time.
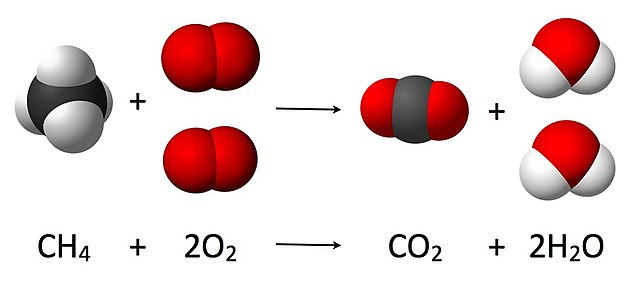
The Combustion reaction of methane. Where 4 atoms of hydrogen, 4 atoms of oxygen, and 1 of carbon are present before and after the reaction. The total mass after the reaction is the same as before the reaction.
Russian scientist Mikhail Lomonosov formulated the law of mass conservation in 1756 and came to the conclusion that the phlogiston theory is incorrect.
Antoine Lavoisier's discovery of the law of conservation of mass led to many new findings in the 19th century. Joseph Proust's law of definite proportions and John Dalton's atomic theory branched from the discoveries of Antoine Lavoisier. Lavoisier's quantitative experiments revealed that combustion involved oxygen rather than what was previously thought to be phlogiston.
Physics is the natural science of matter, involving the study of matter, its fundamental constituents, its motion and behavior through space and time, and the related entities of energy and force. Physics is one of the most fundamental scientific disciplines, with its main goal being to understand how the universe behaves. A scientist who specializes in the field of physics is called a physicist.
Ancient Egyptian astronomy is evident in monuments like the ceiling of Senemut's tomb from the Eighteenth Dynasty of Egypt.
Galileo Galilei (1564–1642) related mathematics, theoretical physics, and experimental physics.
Isaac Newton discovered the laws of motion and universal gravitation
Max Planck (1858–1947), the originator of the theory of quantum mechanics