Direct current (DC) is one-directional flow of electric charge. An electrochemical cell is a prime example of DC power. Direct current may flow through a conductor such as a wire, but can also flow through semiconductors, insulators, or even through a vacuum as in electron or ion beams. The electric current flows in a constant direction, distinguishing it from alternating current (AC). A term formerly used for this type of current was galvanic current.
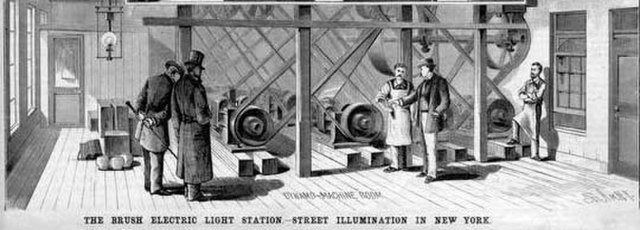
Brush Electric Company's central power plant with dynamos generating direct current to power arc lamps for public lighting in New York. Beginning operation in December 1880 at 133 West Twenty-Fifth Street, the high voltages it operated at allowed it to power a 2-mile (3.2 km) long circuit.
An electrochemical cell is a device that generates electrical energy from chemical reactions. Electrical energy can also be applied to these cells to cause chemical reactions to occur. Electrochemical cells that generate an electric current are called voltaic or galvanic cells and those that generate chemical reactions, via electrolysis for example, are called electrolytic cells.
A modern electrolytic cell consisting of two half reactions, two electrodes, a salt bridge, voltmeter, and a battery.
Circuit diagram of a primary cell showing difference in cell potential, and flow of electrons through a resistor.
Lead acid car battery (secondary cell)