Forensic chemistry is the application of chemistry and its subfield, forensic toxicology, in a legal setting. A forensic chemist can assist in the identification of unknown materials found at a crime scene. Specialists in this field have a wide array of methods and instruments to help identify unknown substances. These include high-performance liquid chromatography, gas chromatography-mass spectrometry, atomic absorption spectroscopy, Fourier transform infrared spectroscopy, and thin layer chromatography. The range of different methods is important due to the destructive nature of some instruments and the number of possible unknown substances that can be found at a scene. Forensic chemists prefer using nondestructive methods first, to preserve evidence and to determine which destructive methods will produce the best results.
A U.S. Customs and Border Protection chemist reads a DNA profile to determine the origin of a commodity
Chemists were able to identify the explosive ANFO at the scene of the Oklahoma City bombing.
A bottle of strychnine extract was once easily obtainable in apothecaries.
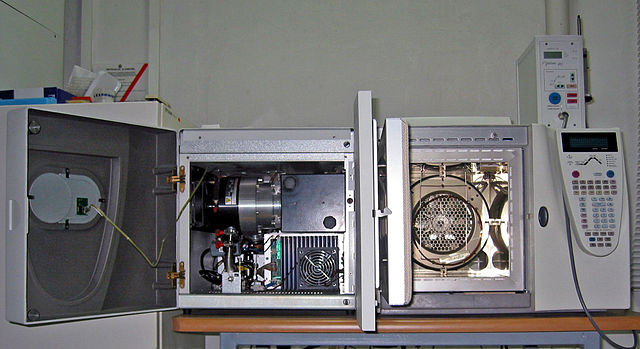
A GC-MS unit with doors open. The gas chromatograph is on the right and the mass spectrometer is on the left.
Jean Servais Stas was a Belgian analytical chemist who co-discovered the atomic weight of carbon.
Stas in an 1894 publication
Nouvelles recherches sur les lois des proportions chimiques : sur les poids atomiques et leurs rapports mutuels (1865)