Free-radical theory of aging
The free radical theory of aging states that organisms age because cells accumulate free radical damage over time. A free radical is any atom or molecule that has a single unpaired electron in an outer shell. While a few free radicals such as melanin are not chemically reactive, most biologically relevant free radicals are highly reactive. For most biological structures, free radical damage is closely associated with oxidative damage. Antioxidants are reducing agents, and limit oxidative damage to biological structures by passivating them from free radicals.
In chemistry, a free radical is any atom, molecule or ion with an unpaired valence electron.
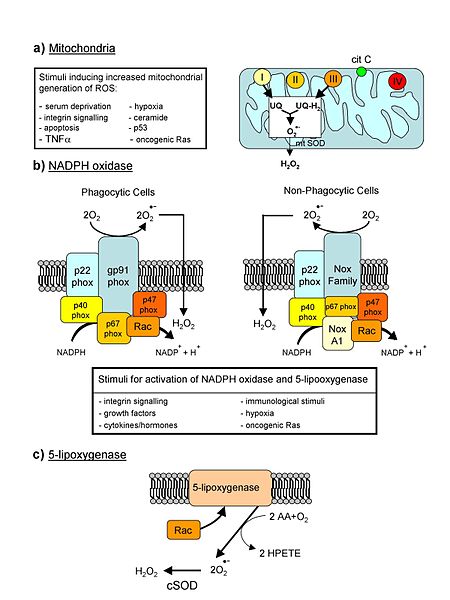
Major sources of reactive oxygen species in living systems
In chemistry, a radical, also known as a free radical, is an atom, molecule, or ion that has at least one unpaired valence electron.
With some exceptions, these unpaired electrons make radicals highly chemically reactive. Many radicals spontaneously dimerize. Most organic radicals have short lifetimes.
The deep colour of lithium naphthalene results from the lithium naphthanide radical.
Moses Gomberg (1866–1947), the founder of radical chemistry