Hemocyanins (also spelled haemocyanins and abbreviated Hc) are proteins that transport oxygen throughout the bodies of some invertebrate animals. These metalloproteins contain two copper atoms that reversibly bind a single oxygen molecule (O2). They are second only to hemoglobin in frequency of use as an oxygen transport molecule. Unlike the hemoglobin in red blood cells found in vertebrates, hemocyanins are not confined in blood cells but are instead suspended directly in the hemolymph. Oxygenation causes a color change between the colorless Cu(I) deoxygenated form and the blue Cu(II) oxygenated form.
Single oxygenated functional unit from the hemocyanin of an octopus
Crystal structure of hexameric haemocyanin from Panulirus interruptus refined at 3.2 angstroms resolution
crystallographic analysis of oxygenated and deoxygenated states of arthropod hemocyanin shows unusual differences
The underside of the carapace of a red rock crab (Cancer productus). The purple coloring is caused by hemocyanin.
Metalloprotein is a generic term for a protein that contains a metal ion cofactor. A large proportion of all proteins are part of this category. For instance, at least 1000 human proteins contain zinc-binding protein domains although there may be up to 3000 human zinc metalloproteins.
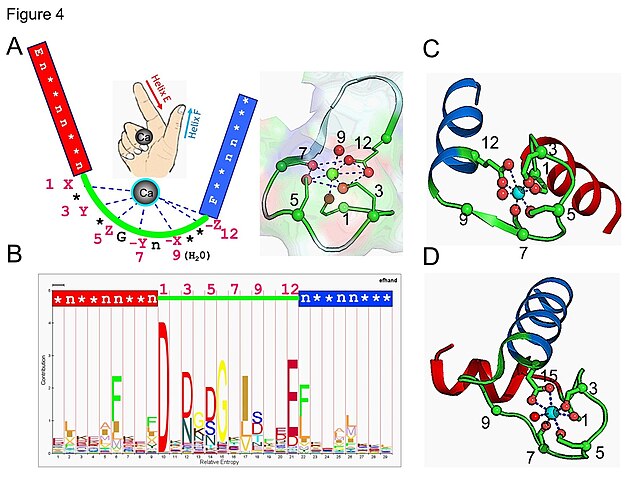
EF-hand motif