In chemistry, a hydrogen bond is primarily an electrostatic force of attraction between a hydrogen (H) atom which is covalently bonded to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing a lone pair of electrons—the hydrogen bond acceptor (Ac). Such an interacting system is generally denoted Dn−H···Ac, where the solid line denotes a polar covalent bond, and the dotted or dashed line indicates the hydrogen bond. The most frequent donor and acceptor atoms are the period 2 elements nitrogen (N), oxygen (O), and fluorine (F).
AFM image of naphthalenetetracarboxylic diimide molecules on silver-terminated silicon, interacting via hydrogen bonding, taken at 77 K. ("Hydrogen bonds" in the top image are exaggerated by artifacts of the imaging technique.)
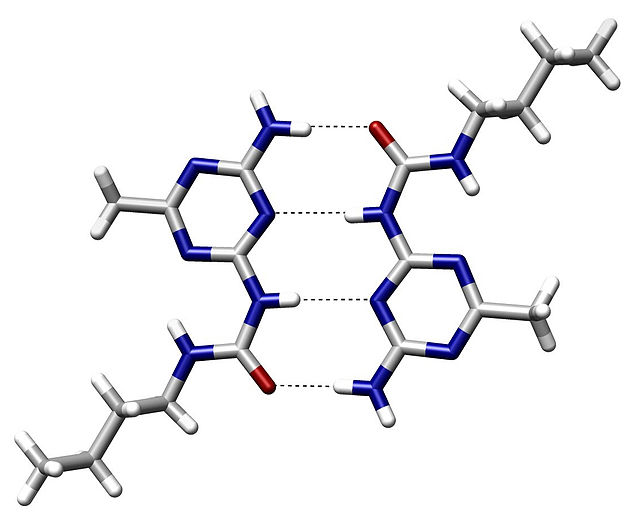
An example of intermolecular hydrogen bonding in a self-assembled dimer complex. The hydrogen bonds are represented by dotted lines.
Chemistry is the scientific study of the properties and behavior of matter. It is a physical science within the natural sciences that studies the chemical elements that make up matter and compounds made of atoms, molecules and ions: their composition, structure, properties, behavior and the changes they undergo during reactions with other substances. Chemistry also addresses the nature of chemical bonds in chemical compounds.
Image: Sulfur sample
Image: Sal (close)
Laboratory, Institute of Biochemistry, University of Cologne in Germany
Solutions of substances in reagent bottles, including ammonium hydroxide and nitric acid, illuminated in different colors