The United States Food and Drug Administration's Investigational New Drug (IND) program is the means by which a pharmaceutical company obtains permission to start human clinical trials and to ship an experimental drug across state lines before a marketing application for the drug has been approved. Regulations are primarily at 21 CFR 312. Similar procedures are followed in the European Union, Japan, and Canada.
Timeline for drug evaluation
The pharmaceutical industry is an industry in medicine that discovers, develops, produces, and markets pharmaceutical drugs for use as medications to be administered to patients, with the aim to cure and prevent diseases, or alleviate symptoms. Pharmaceutical companies may deal in generic or brand medications and medical devices. They are subject to a variety of laws and regulations that govern the patenting, testing, safety, efficacy using drug testing and marketing of drugs. The global pharmaceuticals market produced treatments worth $1,228.45 billion in 2020 and showed a compound annual growth rate (CAGR) of 1.8%.
A drug manufacturer inspection by the US Food and Drug Administration
Diethylbarbituric acid was the first marketed barbiturate. It was sold by Bayer under the trade name Veronal.
In 1937 over 100 people died after ingesting a solution of the antibacterial sulfanilamide formulated in the toxic solvent diethylene glycol.
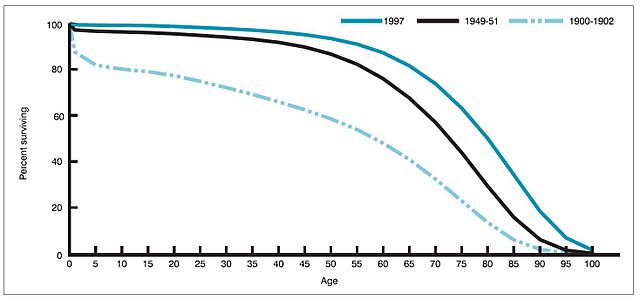
Percent surviving by age in 1900, 1950, and 1997