Kalibangān is a town located at 29.47°N 74.13°E on the left or southern banks of the Ghaggar in Tehsil Pilibangān, between Suratgarh and Hanumangarh in Hanumangarh District, Rajasthan, India 205 km. from Bikaner. It is also identified as being established in the triangle of land at the confluence of Drishadvati and Sarasvati Rivers. The prehistoric and pre-Mauryan character of Indus Valley civilization was first identified by Luigi Tessitori at this site. Kalibangan's excavation report was published in its entirety in 2003 by the Archaeological Survey of India, 34 years after the completion of excavations. The report concluded that Kalibangan was a major provincial capital of the Indus Valley Civilization. Kalibangan is distinguished by its unique fire altars and "world's earliest attested ploughed field". It is around 2900 BC that the region of Kalibangan developed into what can be considered a planned city.
The western mound of Kalibangan, known as the Citadel
Kalibangan pre-Harappan structures
Kalibangan pre-Harappan structural strata
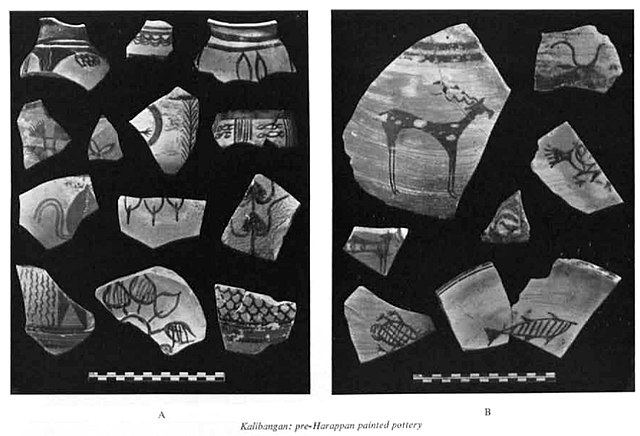
Kalibangan pre-Harappan painted pottery
Suratgarh is a City/Tehsil and a municipality in Sri Ganganagar district and is the biggest tehsil among all the 7 tehsils in Sri Ganganagar district. Suratgarh is also known as Cotton city & Bowl of grain of Rajasthan due to high production level of Cotton and wheat in this area. Suratgarh is 77 Km away in South from it district headquarters just nearby Sri Ganganagar city in Sri Ganganagar district in the Indian state of Rajasthan. Founded by Maharaja Surat Singh. Hindi, Bagri and Rajasthani are the widely spoken languages of the city.
Suratgarh thermal power in Suratgarh, Sri Ganganagar district, Rajasthan, India