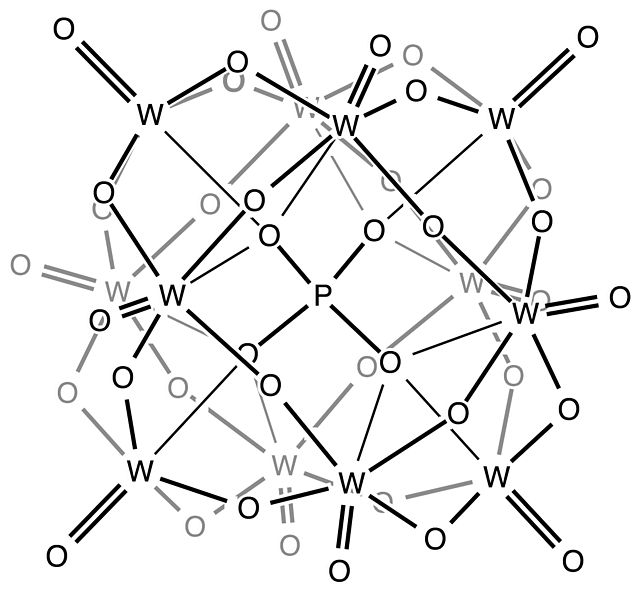
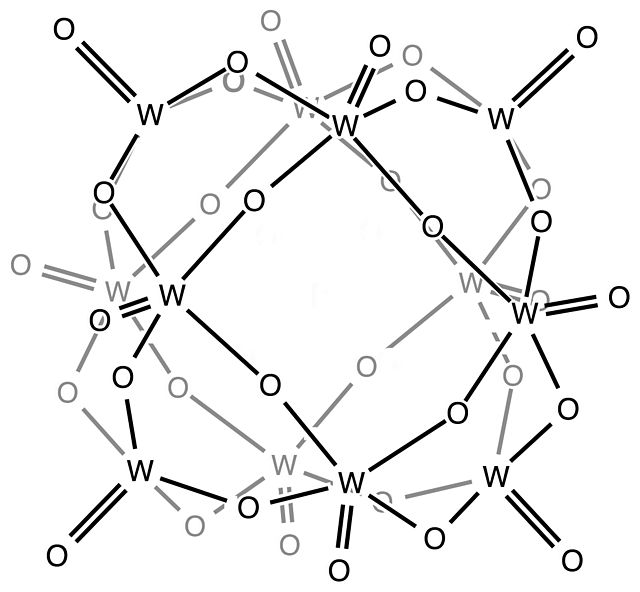
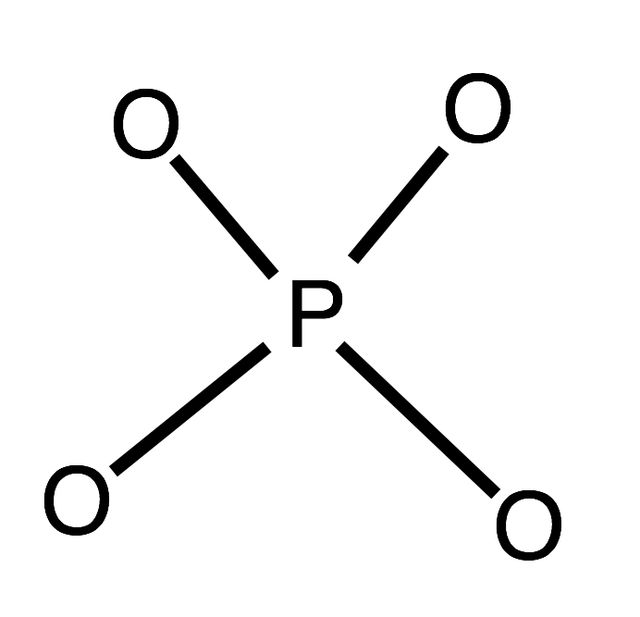
The Keggin structure is the best known structural form for heteropoly acids. It is the structural form of α-Keggin anions, which have a general formula of [XM12O40]n−, where X is the heteroatom, M is the addendum atom, and O represents oxygen. The structure self-assembles in acidic aqueous solution and is a commonly used type of polyoxometalate catalysts.
James F. Keggin, the discoverer of the Keggin Structure.
Image: Kegginstructure
Image: Keggin CAGE
Image: Heteroatom
In chemistry, the heteropolymetalates are a subset of the polyoxometalates, which consist of three or more transition metal oxyanions linked together by shared oxygen atoms to form a closed 3-dimensional molecular framework. In contrast to isopolymetalates, which contain only one kind of metal atom, the heteropolymetalates contain differing main group oxyanions. The metal atoms are usually group 6 or less commonly group 5 transition metals in their highest oxidation states. They are usually colorless to orange, diamagnetic anions. For most heteropolymetalates the W, Mo, or V, is complemented by main group oxyanions phosphate and silicate. Many exceptions to these general statements exist, and the class of compounds includes hundreds of examples.
Heteropolymetalates: K5[IMo6O24]·nH2O Ag7[PV12O36]·nH2O (NH4)4[NiMo6O24H6]·5H2O K3[CrMo6O24H6]·nH2O (NH4)8[CeMo12O42]·8H2O
Image: P2Mo 5
Image: Alfa H3PMo 12O40 30H2O
Image: Dawson color Picture 1


![Heteropolymetalates: K5[IMo6O24]·nH2O Ag7[PV12O36]·nH2O (NH4)4[NiMo6O24H6]·5H2O K3[CrMo6O24H6]·nH2O (NH4)8[CeMo12O42]·8H2O](https://upload.wikimedia.org/wikipedia/commons/thumb/0/05/Heteropolymetalate.%E1%83%95%E1%83%99.jpg/640px-Heteropolymetalate.%E1%83%95%E1%83%99.jpg)


