Limewater is a saturated aqueous solution of calcium hydroxide. Calcium hydroxide, Ca(OH)2, is sparsely soluble at room temperature in water (1.5 g/L at 25 °C). "Pure" (i.e. less than or fully saturated) limewater is clear and colorless, with a slight earthy smell and an astringent/bitter taste. It is basic in nature with a pH of 12.4.
Limewater
Closeup of cast of The Dying Gaul, showing distinctive hairstyle, supposedly derived from washing in limewater.
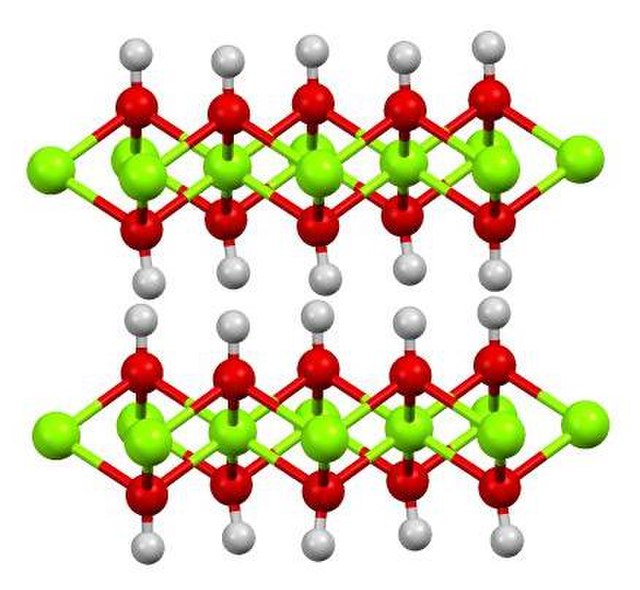
Calcium hydroxide (traditionally called slaked lime) is an inorganic compound with the chemical formula Ca(OH)2. It is a colorless crystal or white powder and is produced when quicklime (calcium oxide) is mixed with water. It has many names including hydrated lime, caustic lime, builders' lime, slaked lime, cal, and pickling lime. Calcium hydroxide is used in many applications, including food preparation, where it has been identified as E number E526. Limewater, also called milk of lime, is the common name for a saturated solution of calcium hydroxide.
Image: Calcium hydroxide
Image: Mg(OH)2Xray
SEM image of fractured hardened cement paste, showing plates of calcium hydroxide and needles of ettringite (micron scale)
Dry untreated maize (left), and treated maize (right) after boiling in water with calcium hydroxide (15 ml, or 1 tbsp, lime for 500 g of corn) for 15 minutes