Sir Marcus Laurence Elwin Oliphant, was an Australian physicist and humanitarian who played an important role in the first experimental demonstration of nuclear fusion and in the development of nuclear weapons.
Oliphant in 1939
The Cavendish Laboratory was the home of some of the great discoveries in physics. It was founded in 1874 by the Duke of Devonshire (Cavendish was his family name), and its first professor was James Clerk Maxwell.
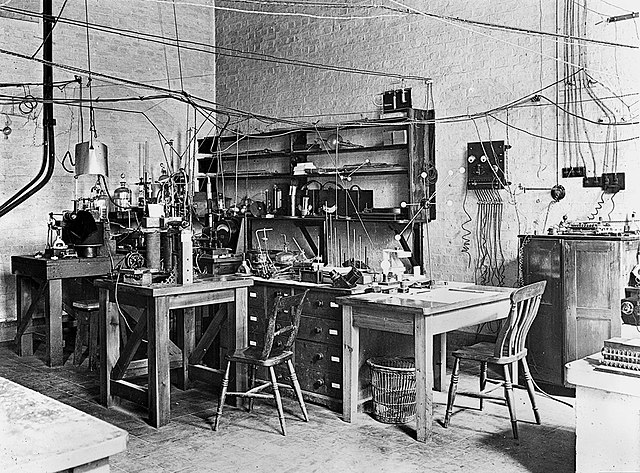
Sir Ernest Rutherford's laboratory, 1926
The Poynting Physics building at the University of Birmingham. Its mode of construction helped give rise to the phrase "redbrick university".
Nuclear fusion is a reaction in which two or more atomic nuclei, usually deuterium and tritium, combine to form one or more different atomic nuclei and subatomic particles. The difference in mass between the reactants and products is manifested as either the release or absorption of energy. This difference in mass arises due to the difference in nuclear binding energy between the atomic nuclei before and after the reaction. Nuclear fusion is the process that powers active or main-sequence stars and other high-magnitude stars, where large amounts of energy are released.
The Sun is a main-sequence star, and thus releases its energy by nuclear fusion of hydrogen nuclei into helium. In its core, the Sun fuses 500 million tonnes of hydrogen each second.
The Tokamak à configuration variable, research fusion reactor, at the École Polytechnique Fédérale de Lausanne (Switzerland).