A permanganate is a chemical compound with the manganate(VII) ion, MnO−4, the conjugate base of permanganic acid. Because the manganese atom has a +7 oxidation state, the permanganate(VII) ion is a strong oxidising agent. The ion is a transition metal ion with a tetrahedral structure. Permanganate solutions are purple in colour and are stable in neutral or slightly alkaline media. The exact chemical reaction depends on the carbon-containing reactants present and the oxidant used. For example, trichloroethane (C2H3Cl3) is oxidised by permanganate ions to form carbon dioxide (CO2), manganese dioxide (MnO2), hydrogen ions (H+), and chloride ions (Cl−).8MnO−4 + 3C2H3Cl3 → 6CO2 + 8MnO2 + H+ + 4H2O + 9Cl−
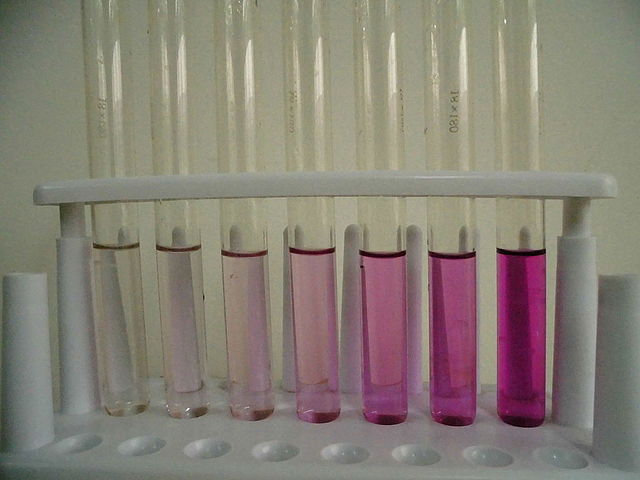
A series of potassium permanganate solutions with varying concentration, increasing to the right.
Manganese is a chemical element; it has symbol Mn and atomic number 25. It is a hard, brittle, silvery metal, often found in minerals in combination with iron. Manganese was first isolated in the 1770s. Manganese is a transition metal with a multifaceted array of industrial alloy uses, particularly in stainless steels. It improves strength, workability, and resistance to wear. Manganese oxide is used as an oxidising agent; as a rubber additive; and in glass making, fertilisers, and ceramics. Manganese sulfate can be used as a fungicide.
Pure manganese cube and oxidized manganese chips
Manganese(II) chloride crystals – the pale pink color of Mn(II) salts is due to a spin-forbidden 3d transition.
Aqueous solution of KMnO4 illustrating the deep purple of Mn(VII) as it occurs in permanganate
Some of the cave paintings in Lascaux, France, use manganese-based pigments.