The phases of ice are all possible states of matter for water as a solid. Currently, 19 phases, including both crystalline and amorphous ice, have been observed at various densities.
The crystal structure of ice XII
An alternative formulation of the phase diagram for certain ices and other phases of water
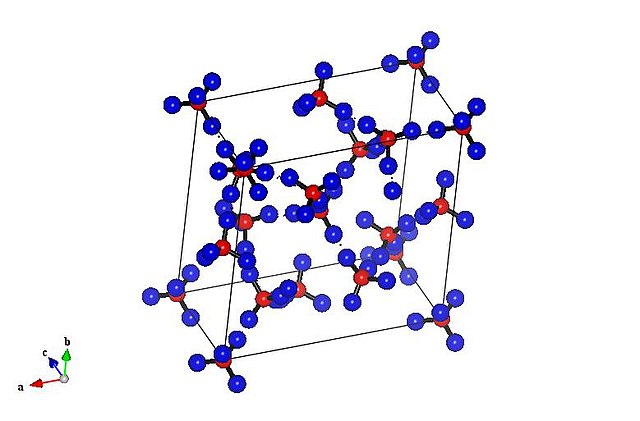
Crystal structure of ice XVII
Photograph showing details of an ice cube under magnification. Ice Ih is the form of ice commonly seen on Earth.
Water is a polar inorganic compound that is at room temperature a tasteless and odorless liquid, which is nearly colorless apart from an inherent hint of blue. It is by far the most studied chemical compound and is described as the "universal solvent" and the "solvent of life". It is the most abundant substance on the surface of Earth and the only common substance to exist as a solid, liquid, and gas on Earth's surface. It is also the third most abundant molecule in the universe.
Properties of water
Dew drops adhering to a spider web
Rain water flux from a canopy. Among the forces that govern drop formation: Surface tension, Cohesion (chemistry), Van der Waals force, Plateau–Rayleigh instability.
This paper clip is under the water level, which has risen gently and smoothly. Surface tension prevents the clip from submerging and the water from overflowing the glass edges.