The plum pudding model is an obsolete scientific model of the atom. It was first proposed by J. J. Thomson in 1904 following his discovery of the electron in 1897, but before Ernest Rutherford discovered the atomic nucleus in 1911. The model tried to account for two properties of atoms then known: that there are electrons and that atoms have no net electric charge. Logically there had to be a commensurate quantity of positive charge to balance out the negative charge of the electrons, but having no clue as to the source of this positive charge, Thomson tentatively proposed it was everywhere in the atom, the atom being in the shape of a sphere. Following from this, Thomson imagined that the balance of electrostatic forces in the atom would distribute the electrons in a more or less even manner throughout this hypothetical sphere.
A 1905 diagram by J. J. Thomson illustrating his proposed arrangements of electrons in an atom, ranging from one to eight electrons.
Thomson used this analogy to visualize the arrangement of electrons in his model of the atom. There are magnetized needles floating in water, oriented such that they repel each other, but are simultaneously attracted to the electromagnet suspended above the center of the pool. This analogy is limited because it is two-dimensional.
Not Thomson's preferred analogy.
Atoms are the basic particles of the chemical elements. An atom consists of a nucleus of protons and generally neutrons, surrounded by an electromagnetically bound swarm of electrons. The chemical elements are distinguished from each other by the number of protons that are in their atoms. For example, any atom that contains 11 protons is sodium, and any atom that contains 29 protons is copper. Atoms with the same number of protons but a different number of neutrons are called isotopes of the same element.
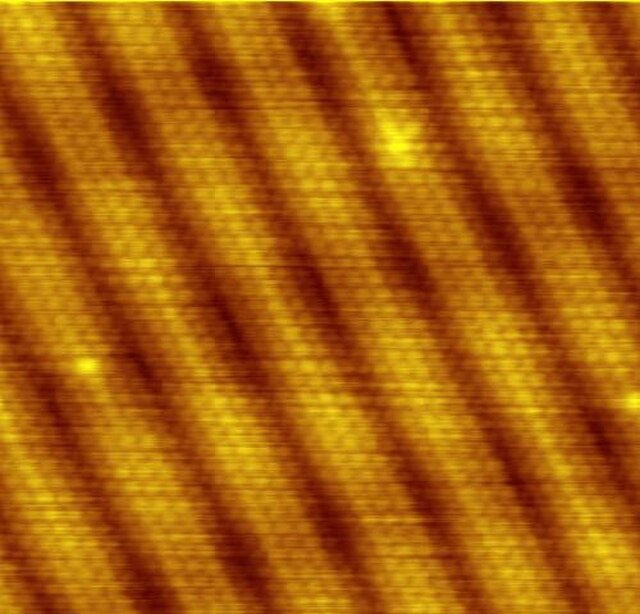
Scanning tunneling microscope image showing the individual atoms making up this gold (100) surface. The surface atoms deviate from the bulk crystal structure and arrange in columns several atoms wide with pits between them (See surface reconstruction).