In chemistry, a salt or ionic compound is a chemical compound consisting of an ionic assembly of positively charged cations and negatively charged anions, which results in a neutral compound with no net electric charge. The constituent ions are held together by electrostatic forces termed ionic bonds.
X-ray spectrometer developed by W. H. Bragg
Halite, the mineral form of sodium chloride, forms when salty water evaporates leaving the ions behind.
Solid lead(II) sulfate (PbSO4)
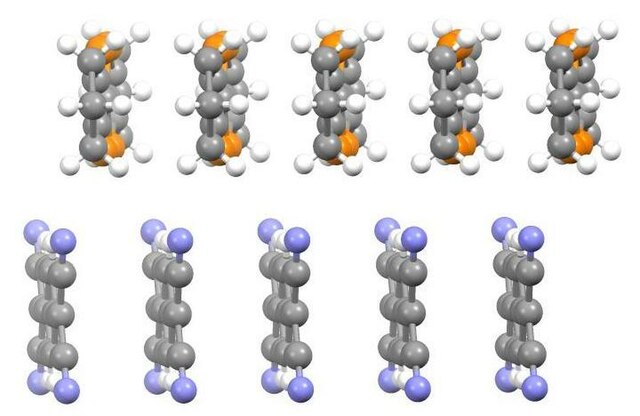
Edge-on view of portion of crystal structure of hexamethyleneTTF/TCNQ charge transfer salt.
Chemistry is the scientific study of the properties and behavior of matter. It is a physical science within the natural sciences that studies the chemical elements that make up matter and compounds made of atoms, molecules and ions: their composition, structure, properties, behavior and the changes they undergo during reactions with other substances. Chemistry also addresses the nature of chemical bonds in chemical compounds.
Image: Sulfur sample
Image: Sal (close)
Laboratory, Institute of Biochemistry, University of Cologne in Germany
Solutions of substances in reagent bottles, including ammonium hydroxide and nitric acid, illuminated in different colors