Slag is a by-product of smelting (pyrometallurgical) ores and recycled metals. Slag is mainly a mixture of metal oxides and silicon dioxide. Broadly, it can be classified as ferrous, ferroalloy or non-ferrous/base metals. Within these general categories, slags can be further categorized by their precursor and processing conditions. "Slag generated from the EAF process can contain toxic metals, which can be hazardous to human and environmental health".
Molten slag is carried outside and poured into a dump
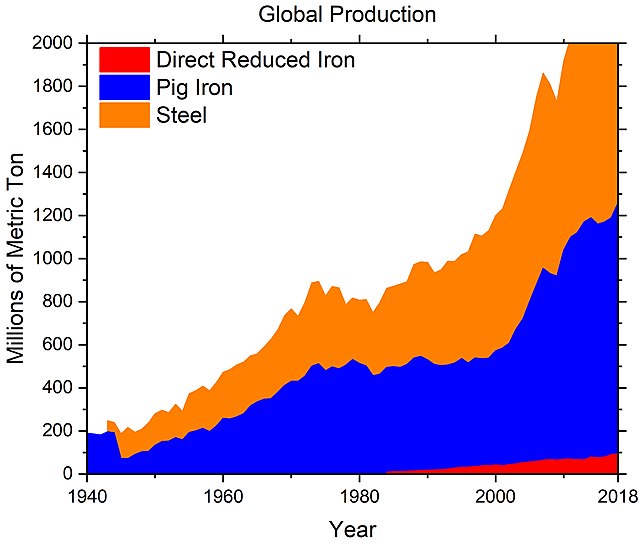
Global production of iron and steel, 1942–2018, according to USGS.
The Manufacture of Iron – Carting Away the Scoriæ (slag), an 1873 wood engraving
Slag run-off from one of the open hearth furnaces of a steel mill, Republic Steel, Youngstown, Ohio, November 1941. Slag is drawn off the furnace just before the molten steel is poured into ladles for ingotting.
Smelting is a process of applying heat and a chemical reducing agent to an ore to extract a desired base metal product. It is a form of extractive metallurgy that is used to obtain many metals such as iron, copper, silver, tin, lead and zinc. Smelting uses heat and a chemical reducing agent to decompose the ore, driving off other elements as gases or slag and leaving the metal behind. The reducing agent is commonly a fossil fuel source of carbon, such as carbon monoxide from incomplete combustion of coke—or, in earlier times, of charcoal. The oxygen in the ore binds to carbon at high temperatures as the chemical potential energy of the bonds in carbon dioxide is lower than that of the bonds in the ore.
Electric phosphate smelting furnace in a TVA chemical plant (1942)
Copper smelter, Chelyabinsk Oblast, Russia
Electrolytic cells at an aluminum smelter in Saint-Jean-de-Maurienne, France
Cowles Syndicate of Ohio in Stoke-upon-Trent England, late 1880s. British Aluminium used the process of Paul Héroult about this time.