Supercritical water oxidation
Supercritical water oxidation (SCWO) is a process that occurs in water at temperatures and pressures above a mixture's thermodynamic critical point. Under these conditions water becomes a fluid with unique properties that can be used to advantage in the destruction of recalcitrant and hazardous wastes such as polychlorinated biphenyls (PCB) or per- and polyfluoroalkyl substances (PFAS). Supercritical water has a density between that of water vapor and liquid at standard conditions, and exhibits high gas-like diffusion rates along with high liquid-like collision rates. In addition, the behavior of water as a solvent is altered - it behaves much less like a polar solvent. As a result, the solubility behavior is "reversed" so that oxygen, and organics such as chlorinated hydrocarbons become soluble in the water, allowing single-phase reaction of aqueous waste with a dissolved oxidizer. The reversed solubility also causes salts to precipitate out of solution, meaning they can be treated using conventional methods for solid-waste residuals. Efficient oxidation reactions occur at low temperature with reduced NOx production.

Supercritical Water (Red Area)
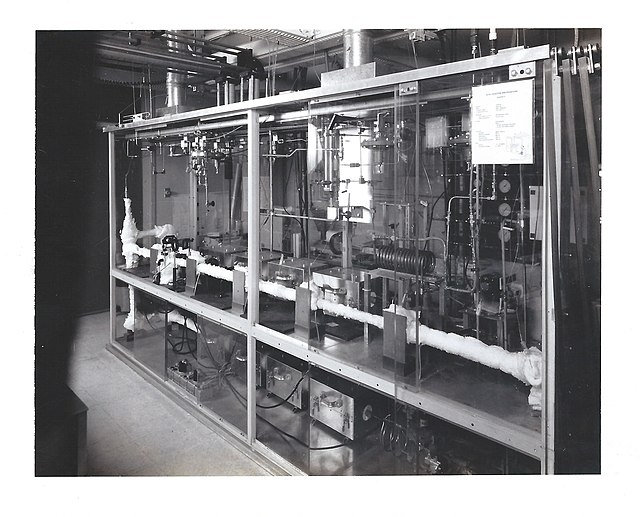
The supercritical fluids reactor (SFR) at Sandia National Laboratories' Combustion Research Facility (CRF), 1995.
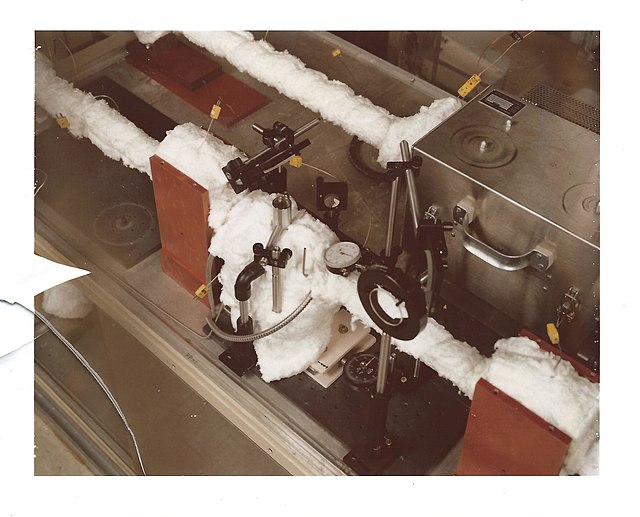
High-pressure, high-temperature optical cell.
Critical point (thermodynamics)
In thermodynamics, a critical point is the end point of a phase equilibrium curve. One example is the liquid–vapor critical point, the end point of the pressure–temperature curve that designates conditions under which a liquid and its vapour can coexist. At higher temperatures, the gas cannot be liquefied by pressure alone. At the critical point, defined by a critical temperature Tc and a critical pressure pc, phase boundaries vanish. Other examples include the liquid–liquid critical points in mixtures, and the ferromagnet–paramagnet transition in the absence of an external magnetic field.
Subcritical ethane, liquid and gas phase coexist. Critical point (32.17 °C, 48.72 bar), opalescence. Supercritical ethane, fluid.
Critical carbon dioxide exuding fog while cooling from supercritical to critical temperature.