Lab-grown diamond is diamond that is produced in a controlled technological process. Unlike diamond simulants, synthetic diamonds are composed of the same material as naturally formed diamonds—pure carbon crystallized in an isotropic 3D form—and share identical chemical and physical properties.
Lab-grown diamonds of various colors grown by the high-pressure-and-temperature technique
Synthetic diamonds, which have a different shade due to the different content of nitrogen impurities. Yellow diamonds are obtained with a higher nitrogen content in the carbon lattice, and transparent diamonds come only from pure carbon. The smallest yellow diamond size is around 0,3 mm
Moissan trying to create synthetic diamonds using an electric arc furnace
First synthetic diamonds by ASEA 1953
Diamond is a solid form of the element carbon with its atoms arranged in a crystal structure called diamond cubic. Another solid form of carbon known as graphite is the chemically stable form of carbon at room temperature and pressure, but diamond is metastable and converts to it at a negligible rate under those conditions. Diamond has the highest hardness and thermal conductivity of any natural material, properties that are used in major industrial applications such as cutting and polishing tools. They are also the reason that diamond anvil cells can subject materials to pressures found deep in the Earth.
A naturally-cut diamond crystal
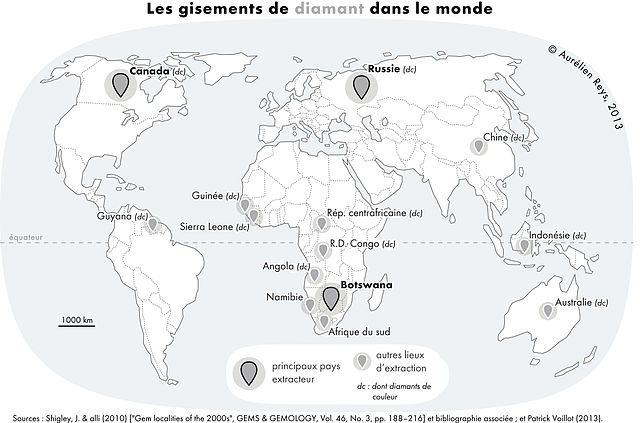
Main diamond producing countries
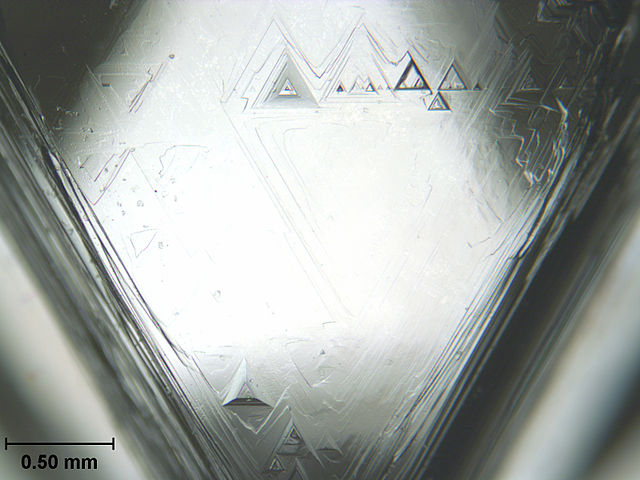
One face of an uncut octahedral diamond, showing trigons (of positive and negative relief) formed by natural chemical etching
The extreme hardness of diamond in certain orientations makes it useful in materials science, as in this pyramidal diamond embedded in the working surface of a Vickers hardness tester.