Thermodynamics is a branch of physics that deals with heat, work, and temperature, and their relation to energy, entropy, and the physical properties of matter and radiation. The behavior of these quantities is governed by the four laws of thermodynamics, which convey a quantitative description using measurable macroscopic physical quantities, but may be explained in terms of microscopic constituents by statistical mechanics. Thermodynamics applies to a wide variety of topics in science and engineering, especially physical chemistry, biochemistry, chemical engineering and mechanical engineering, but also in other complex fields such as meteorology.
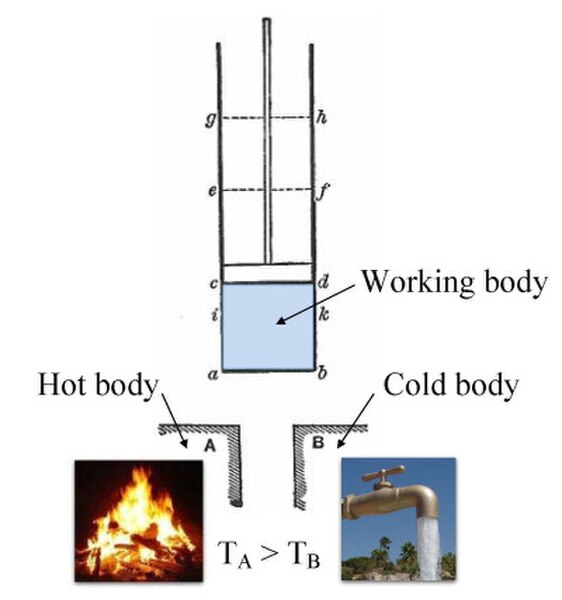
Annotated color version of the original 1824 Carnot heat engine showing the hot body (boiler), working body (system, steam), and cold body (water), the letters labeled according to the stopping points in Carnot cycle
Opening a bottle of sparkling wine (high-speed photography). The sudden drop of pressure causes a huge drop of temperature. The moisture in the air freezes, creating a smoke of tiny ice crystals.
Physics is the natural science of matter, involving the study of matter, its fundamental constituents, its motion and behavior through space and time, and the related entities of energy and force. Physics is one of the most fundamental scientific disciplines, with its main goal being to understand how the universe behaves. A scientist who specializes in the field of physics is called a physicist.
Ancient Egyptian astronomy is evident in monuments like the ceiling of Senemut's tomb from the Eighteenth Dynasty of Egypt.
Galileo Galilei (1564–1642) related mathematics, theoretical physics, and experimental physics.
Isaac Newton discovered the laws of motion and universal gravitation
Max Planck (1858–1947), the originator of the theory of quantum mechanics