Viral neuraminidase is a type of neuraminidase found on the surface of influenza viruses that enables the virus to be released from the host cell. Neuraminidases are enzymes that cleave sialic acid groups from glycoproteins. Viral neuraminidase was discovered by Alfred Gottschalk at the Walter and Eliza Hall Institute in 1957. Neuraminidase inhibitors are antiviral agents that inhibit influenza viral neuraminidase activity and are of major importance in the control of influenza.
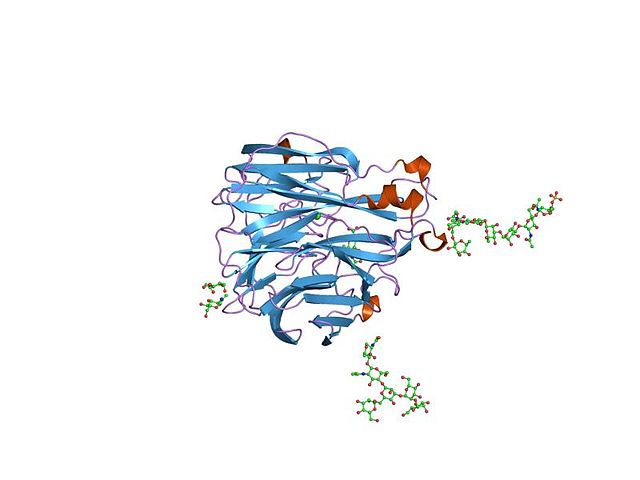
Crystallographic structure of influenza neuraminidase in complex with the inhibitor 4-acetamido-3-hydroxy-5-nitro-benzoic acid.
The structure of the influenza virus neuraminidase.
Structure of Influenza, showing neuraminidase marked as NA and hemagglutinin as HA
Exo-α-sialidase is a glycoside hydrolase that cleaves the glycosidic linkages of neuraminic acids:Hydrolysis of α-(2→3)-, α-(2→6)-, α-(2→8)- glycosidic linkages of terminal sialic acid residues in oligosaccharides, glycoproteins, glycolipids, colominic acid and synthetic substrates
Neuraminidase (GH34) ribbon diagram. An analog of its neuraminic acid substrate, used as an inhibitor drug, is the small white and red molecule in the center.