In organic chemistry, an alkane, or paraffin, is an acyclic saturated hydrocarbon. In other words, an alkane consists of hydrogen and carbon atoms arranged in a tree structure in which all the carbon–carbon bonds are single. Alkanes have the general chemical formula CnH2n+2. The alkanes range in complexity from the simplest case of methane, where n = 1, to arbitrarily large and complex molecules, like pentacontane or 6-ethyl-2-methyl-5-(1-methylethyl) octane, an isomer of tetradecane.
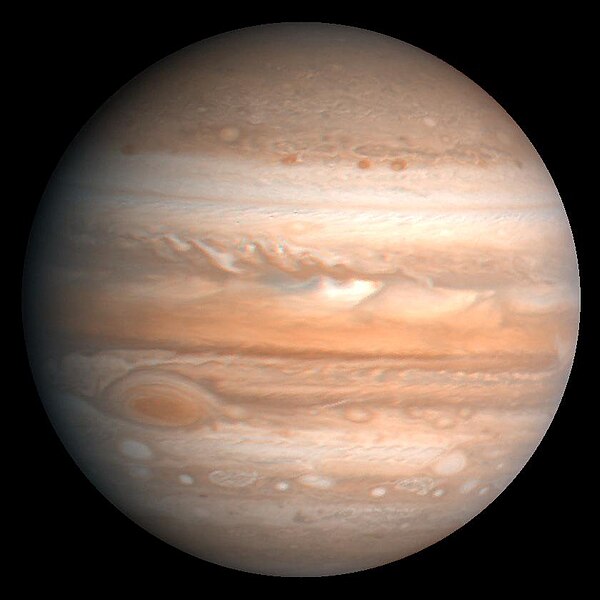
Methane and ethane make up a tiny proportion of Jupiter's atmosphere
Extraction of oil, which contains many distinct hydrocarbons including alkanes
Methanogenic archaea in the gut of cows produce methane.
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and hydrophobic; their odor is usually faint, and may be similar to that of gasoline or lighter fluid. They occur in a diverse range of molecular structures and phases: they can be gases, liquids, low melting solids or polymers.
Oil refineries are one way hydrocarbons are processed for use. Crude oil is processed in several stages to form desired hydrocarbons, used as fuel and in other products.
Tank wagon 33 80 7920 362-0 with hydrocarbon gas at Bahnhof Enns (2018)
Natural oil spring in Korňa, Slovakia