An alum is a type of chemical compound, usually a hydrated double sulfate salt of aluminium with the general formula XAl(SO4)2·12 H2O, such that X is a monovalent cation such as potassium or ammonium. By itself, "alum" often refers to potassium alum, with the formula KAl(SO4)2·12 H2O. Other alums are named after the monovalent ion, such as sodium alum and ammonium alum.
Crystal of potassium alum, KAl(SO 4) 2·12H 2O
Crystal of potassium alum.
Chrome alum crystal.
Alum crystal with small amount of chrome alum to give a slight violet color.
The sulfate or sulphate ion is a polyatomic anion with the empirical formula SO2−4. Salts, acid derivatives, and peroxides of sulfate are widely used in industry. Sulfates occur widely in everyday life. Sulfates are salts of sulfuric acid and many are prepared from that acid.
Knapsack sprayer used to apply sulfate to vegetables. Valencian Museum of Ethnology.
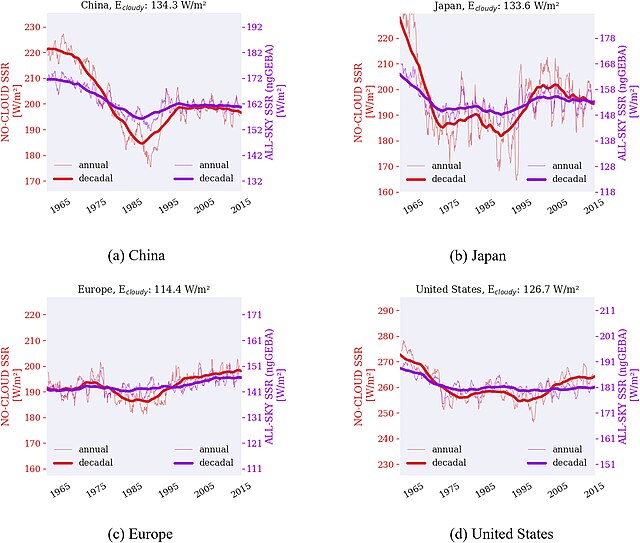
The observed trends of global dimming and brightening in four major geographic regions. The dimming was greater on the average cloud-free days (red line) than on the average of all days (purple line), strongly suggesting that sulfate aerosols were the cause.
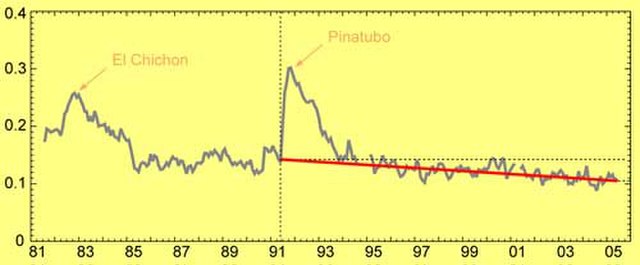
Sun-blocking aerosols around the world steadily declined (red line) since the 1991 eruption of Mount Pinatubo, according to satellite estimates.
Satellite photo showing a thick pall of smoke and haze from forest fires in Eastern China. Such smoke is full of black carbon, which contributes to dimming trends but has an overall warming effect.