The sulfate or sulphate ion is a polyatomic anion with the empirical formula SO2−4. Salts, acid derivatives, and peroxides of sulfate are widely used in industry. Sulfates occur widely in everyday life. Sulfates are salts of sulfuric acid and many are prepared from that acid.
Knapsack sprayer used to apply sulfate to vegetables. Valencian Museum of Ethnology.
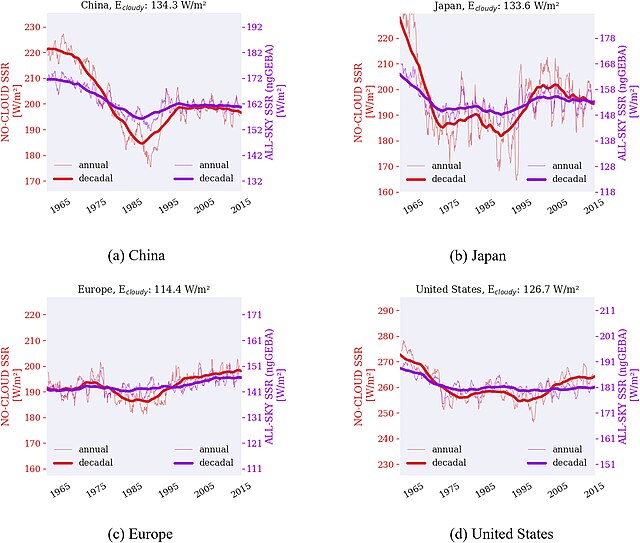
The observed trends of global dimming and brightening in four major geographic regions. The dimming was greater on the average cloud-free days (red line) than on the average of all days (purple line), strongly suggesting that sulfate aerosols were the cause.
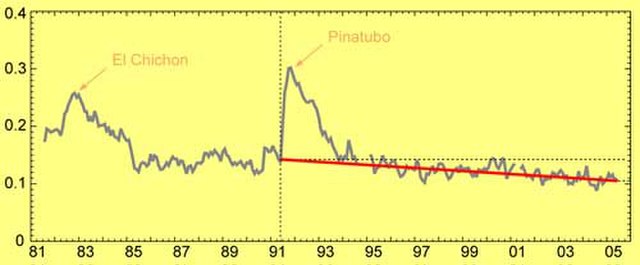
Sun-blocking aerosols around the world steadily declined (red line) since the 1991 eruption of Mount Pinatubo, according to satellite estimates.
Satellite photo showing a thick pall of smoke and haze from forest fires in Eastern China. Such smoke is full of black carbon, which contributes to dimming trends but has an overall warming effect.
In chemistry, a salt or ionic compound is a chemical compound consisting of an ionic assembly of positively charged cations and negatively charged anions, which results in a neutral compound with no net electric charge. The constituent ions are held together by electrostatic forces termed ionic bonds.
X-ray spectrometer developed by W. H. Bragg
Halite, the mineral form of sodium chloride, forms when salty water evaporates leaving the ions behind.
Solid lead(II) sulfate (PbSO4)
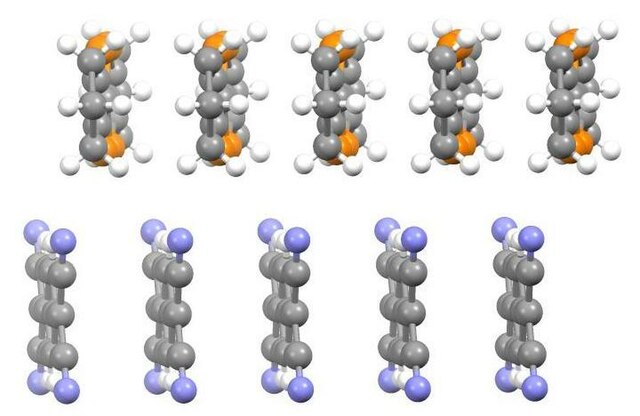
Edge-on view of portion of crystal structure of hexamethyleneTTF/TCNQ charge transfer salt.