Amyloids are aggregates of proteins characterised by a fibrillar morphology of typically 7–13 nm in diameter, a β-sheet secondary structure and ability to be stained by particular dyes, such as Congo red. In the human body, amyloids have been linked to the development of various diseases. Pathogenic amyloids form when previously healthy proteins lose their normal structure and physiological functions (misfolding) and form fibrous deposits within and around cells. These protein misfolding and deposition processes disrupt the healthy function of tissues and organs.
Micrograph showing amyloid deposits (pink) in small bowel. Duodenum with amyloid deposition in lamina propria. Amyloid shows up as homogeneous pink material in lamina propria and around blood vessels. 20× magnification.
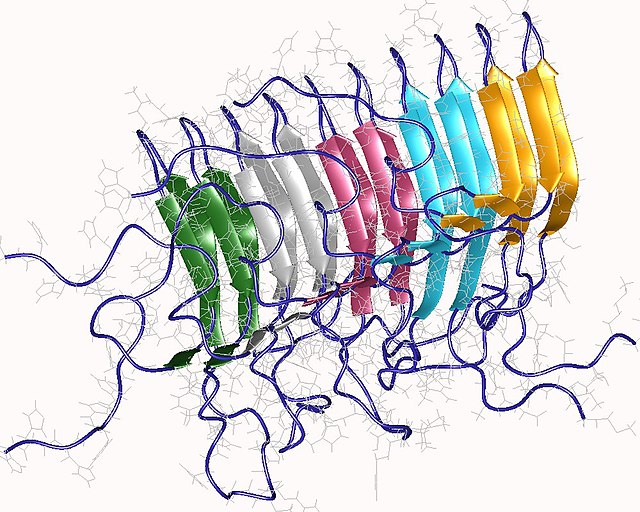
Amyloid of HET-s(218-289) prion pentamer, Podospora anserina (PDB: 2rnm)
Structure of a fibril, consisting of one single protofilament, of the amyloid β peptide viewed down the long axis of the fibril (PDB: 2mlq)
The beta sheet is a common motif of the regular protein secondary structure. Beta sheets consist of beta strands (β-strands) connected laterally by at least two or three backbone hydrogen bonds, forming a generally twisted, pleated sheet. A β-strand is a stretch of polypeptide chain typically 3 to 10 amino acids long with backbone in an extended conformation. The supramolecular association of β-sheets has been implicated in the formation of the fibrils and protein aggregates observed in amyloidosis, Alzheimer's disease and other proteinopathies.
Ramachandran (φ, ψ) plot of about 100,000 high-resolution data points, showing the broad, favorable region around the conformation typical for β-sheet amino acid residues.
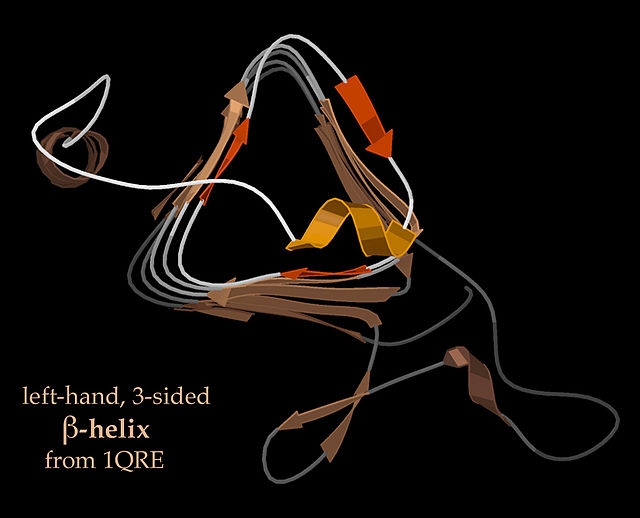
End-view of a 3-sided, left handed β-helix (PDB: 1QRE)
End-view of a 3-sided, right-handed β-helix (PDB: 2PEC)