The beta sheet is a common motif of the regular protein secondary structure. Beta sheets consist of beta strands (β-strands) connected laterally by at least two or three backbone hydrogen bonds, forming a generally twisted, pleated sheet. A β-strand is a stretch of polypeptide chain typically 3 to 10 amino acids long with backbone in an extended conformation. The supramolecular association of β-sheets has been implicated in the formation of the fibrils and protein aggregates observed in amyloidosis, Alzheimer's disease and other proteinopathies.
Ramachandran (φ, ψ) plot of about 100,000 high-resolution data points, showing the broad, favorable region around the conformation typical for β-sheet amino acid residues.
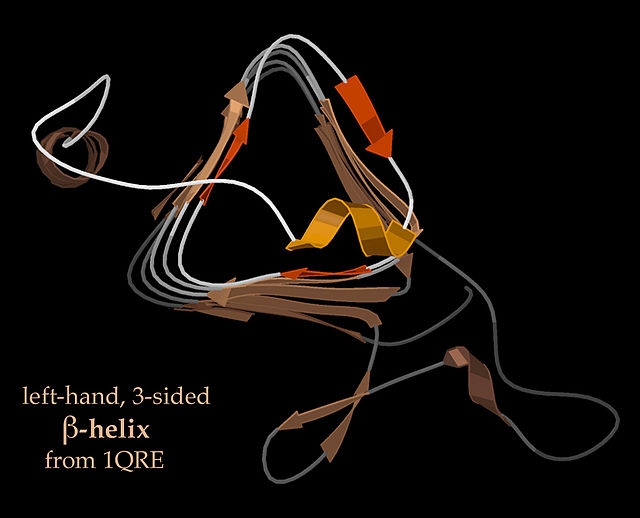
End-view of a 3-sided, left handed β-helix (PDB: 1QRE)
End-view of a 3-sided, right-handed β-helix (PDB: 2PEC)
In chemistry, a hydrogen bond is primarily an electrostatic force of attraction between a hydrogen (H) atom which is covalently bonded to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing a lone pair of electrons—the hydrogen bond acceptor (Ac). Such an interacting system is generally denoted Dn−H···Ac, where the solid line denotes a polar covalent bond, and the dotted or dashed line indicates the hydrogen bond. The most frequent donor and acceptor atoms are the period 2 elements nitrogen (N), oxygen (O), and fluorine (F).
AFM image of naphthalenetetracarboxylic diimide molecules on silver-terminated silicon, interacting via hydrogen bonding, taken at 77 K. ("Hydrogen bonds" in the top image are exaggerated by artifacts of the imaging technique.)
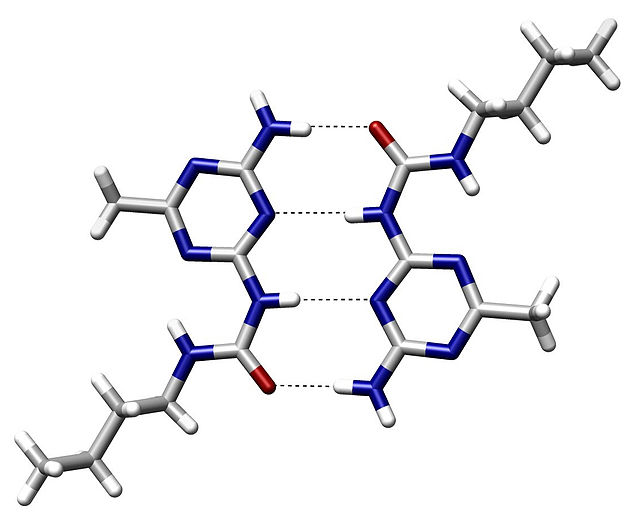
An example of intermolecular hydrogen bonding in a self-assembled dimer complex. The hydrogen bonds are represented by dotted lines.