An anode is an electrode of a polarized electrical device through which conventional current enters the device. This contrasts with a cathode, an electrode of the device through which conventional current leaves the device. A common mnemonic is ACID, for "anode current into device". The direction of conventional current in a circuit is opposite to the direction of electron flow, so electrons flow from the anode of a galvanic cell, into an outside or external circuit connected to the cell. For example, the end of a household battery marked with a "+" is the cathode.
Sacrificial anodes mounted "on the fly" for corrosion protection of a metal structure
An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit. Electrodes are essential parts of batteries that can consist of a variety of materials depending on the type of battery.
Electrodes used in shielded metal arc welding
Various disposable batteries: two 9-volt, two "AAA", two "AA", and one each of "C", "D", a cordless phone battery, a camcorder battery, a 2-meter handheld ham radio battery, and a button battery.
Rechargeable Batteries
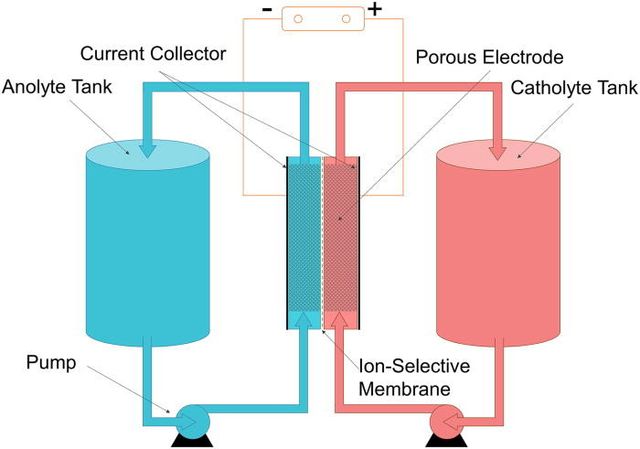
A typical flow battery consists of two tanks of liquids which are pumped past a membrane held between two electrodes.