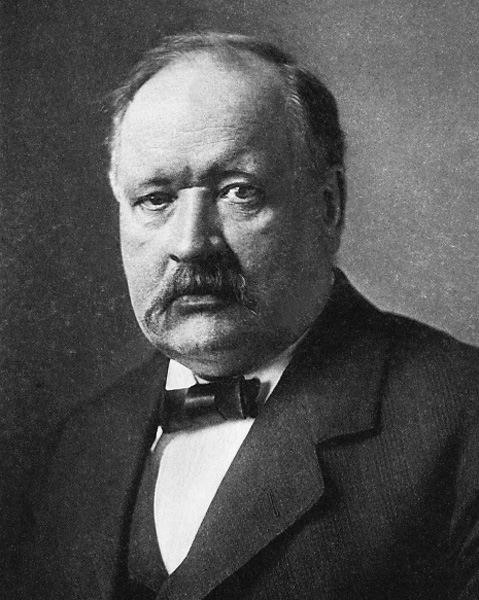An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit. Electrodes are essential parts of batteries that can consist of a variety of materials depending on the type of battery.
Electrodes used in shielded metal arc welding
Various disposable batteries: two 9-volt, two "AAA", two "AA", and one each of "C", "D", a cordless phone battery, a camcorder battery, a 2-meter handheld ham radio battery, and a button battery.
Rechargeable Batteries
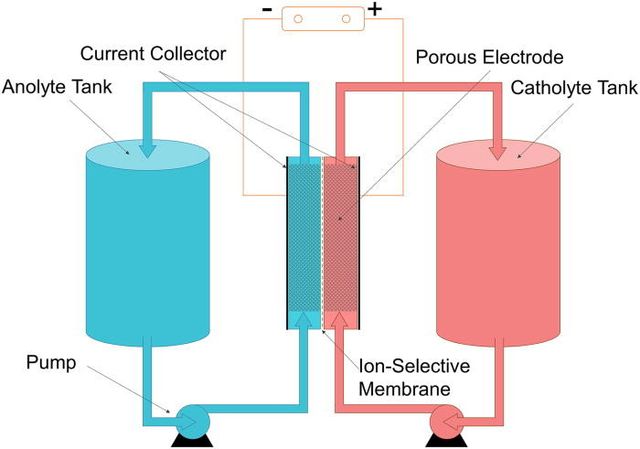
A typical flow battery consists of two tanks of liquids which are pumped past a membrane held between two electrodes.
An electrolyte is a medium containing ions that are electrically conductive through the movement of those ions, but not conducting electrons. This includes most soluble salts, acids, and bases dissolved in a polar solvent, such as water. Upon dissolving, the substance separates into cations and anions, which disperse uniformly throughout the solvent. Solid-state electrolytes also exist. In medicine and sometimes in chemistry, the term electrolyte refers to the substance that is dissolved.
Svante Arrhenius, father of the concept of electrolyte dissociation in aqueous solution for which he received the Nobel Prize in Chemistry in 1903