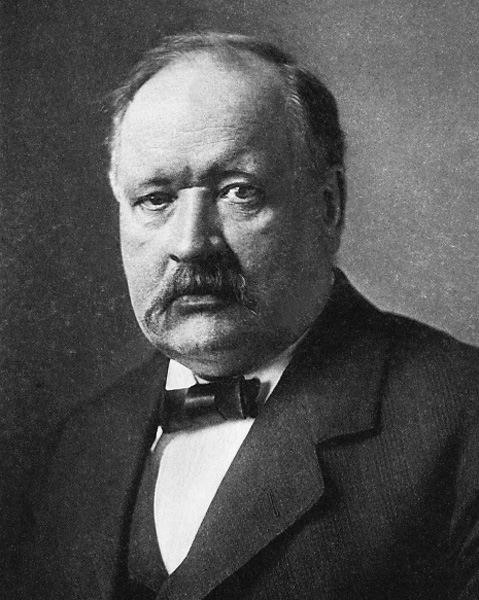An electrolyte is a medium containing ions that are electrically conductive through the movement of those ions, but not conducting electrons. This includes most soluble salts, acids, and bases dissolved in a polar solvent, such as water. Upon dissolving, the substance separates into cations and anions, which disperse uniformly throughout the solvent. Solid-state electrolytes also exist. In medicine and sometimes in chemistry, the term electrolyte refers to the substance that is dissolved.
Svante Arrhenius, father of the concept of electrolyte dissociation in aqueous solution for which he received the Nobel Prize in Chemistry in 1903
In chemistry, a salt or ionic compound is a chemical compound consisting of an ionic assembly of positively charged cations and negatively charged anions, which results in a neutral compound with no net electric charge. The constituent ions are held together by electrostatic forces termed ionic bonds.
X-ray spectrometer developed by W. H. Bragg
Halite, the mineral form of sodium chloride, forms when salty water evaporates leaving the ions behind.
Solid lead(II) sulfate (PbSO4)
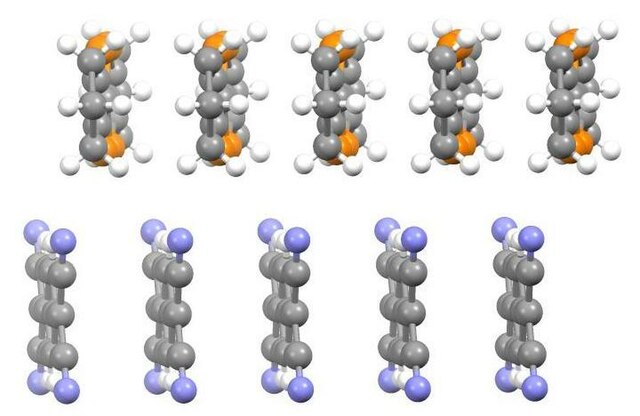
Edge-on view of portion of crystal structure of hexamethyleneTTF/TCNQ charge transfer salt.