Coniine is a poisonous chemical compound, an alkaloid present in and isolable from poison hemlock (Conium maculatum), where its presence has been a source of significant economic, medical, and historico-cultural interest; coniine is also produced by the yellow pitcher plant (Sarracenia flava), and fool's parsley (Aethusa cynapium). Its ingestion and extended exposure are toxic to humans and all classes of livestock; its mechanism of poisoning involves disruption of the central nervous system, with death caused by respiratory paralysis. The biosynthesis of coniine contains as its penultimate step the non-enzymatic cyclisation of 5-oxooctylamine to γ-coniceine, a Schiff base differing from coniine only by its carbon-nitrogen double bond in the ring. This pathway results in natural coniine that is a mixture—a racemate—composed of two enantiomers, the stereoisomers (S)-(+)-coniine and (R)-(−)-coniine, depending on the direction taken by the chain that branches from the ring. Both enantiomers are toxic, with the (R)-enantiomer being the more biologically active and toxic of the two in general. Coniine holds a place in organic chemistry history as being the first of the important class of alkaloids to be synthesized, by Albert Ladenburg in 1886, and it has been synthesized in the laboratory in a number of unique ways through to modern times.

The poison hemlock plant.
The yellow pitcher plant
Alkaloids are a class of basic, naturally occurring organic compounds that contain at least one nitrogen atom. This group also includes some related compounds with neutral and even weakly acidic properties. Some synthetic compounds of similar structure may also be termed alkaloids. In addition to carbon, hydrogen and nitrogen, alkaloids may also contain oxygen or sulfur. More rarely still, they may contain elements such as phosphorus, chlorine, and bromine.
The first individual alkaloid, morphine, was isolated in 1804 from the opium poppy (Papaver somniferum).
Friedrich Sertürner, the German chemist who first isolated morphine from opium.
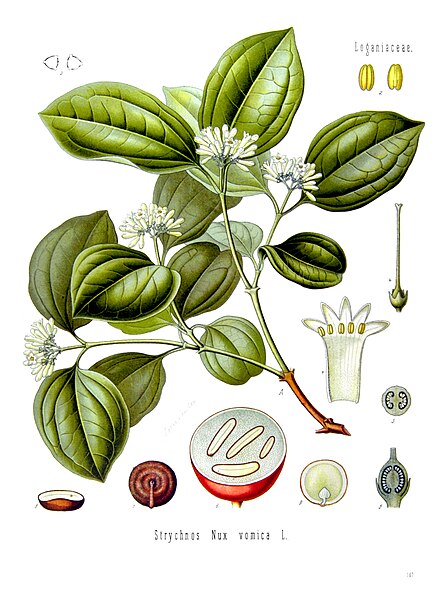
Strychnine tree. Its seeds are rich in strychnine and brucine.
Crystals of piperine extracted from black pepper.