A refrigerator designed to reach cryogenic temperatures is often called a cryocooler. The term is most often used for smaller systems, typically table-top size, with input powers less than about 20 kW. Some can have input powers as low as 2–3 W. Large systems, such as those used for cooling the superconducting magnets in particle accelerators are more often called cryogenic refrigerators. Their input powers can be as high as 1 MW. In most cases cryocoolers use a cryogenic fluid as the working substance and employ moving parts to cycle the fluid around a thermodynamic cycle. The fluid is typically compressed at room temperature, precooled in a heat exchanger, then expanded at some low temperature. The returning low-pressure fluid passes through the heat exchanger to precool the high-pressure fluid before entering the compressor intake. The cycle is then repeated.
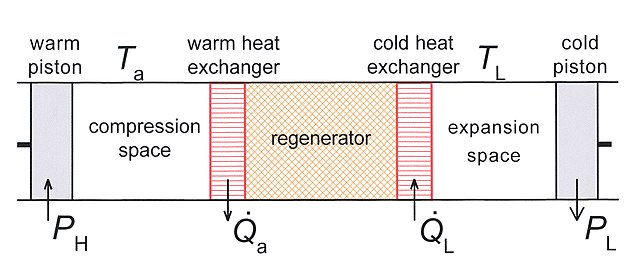
Fig.1 Schematic diagram of a Stirling cooler. The system has one piston at ambient temperature Ta and one piston at low temperature TL.
Fig.2 Four states in the Stirling cycle.
Fig.3 pV-diagram of the ideal Stirling cycle.
Fig.4 Schematic diagram of a split-pair Stirling refrigerator. The cooling power is supplied to the heat exchanger of the cold finger. Usually the heat flows are so small that there is no need for physical heat exchangers around the split pipe.
In physics, cryogenics is the production and behaviour of materials at very low temperatures.
Nitrogen is a liquid under −195.8 °C (77.3 K).
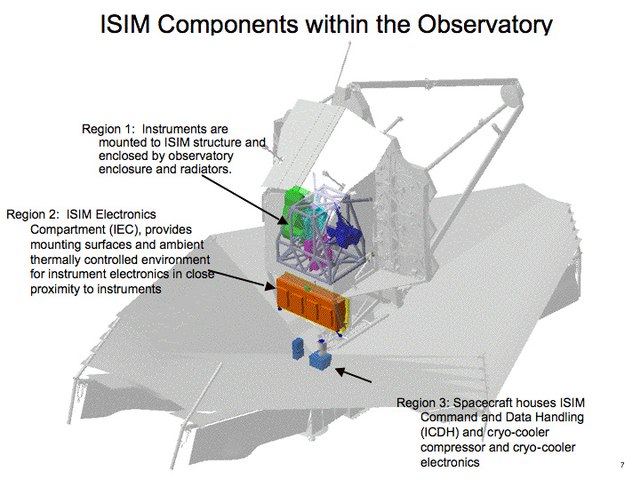
This is a diagram of an infrared space telescope, that needs a cold mirror and instruments. One instrument needs to be even colder, and it has a cryocooler. The instrument is in region 1 and its cryocooler is in region 3 in a warmer region of the spacecraft (see MIRI (Mid-Infrared Instrument) or James Webb Space Telescope).
A medium-sized dewar is being filled with liquid nitrogen by a larger cryogenic storage tank.
Catalogue image of a cryogenic valve