Deflagration is subsonic combustion in which a pre-mixed flame propagates through an explosive or a mixture of fuel and oxidizer. Deflagrations in high and low explosives or fuel–oxidizer mixtures may transition to a detonation depending upon confinement and other factors. Most fires found in daily life are diffusion flames. Deflagrations with flame speeds in the range of 1 m/s differ from detonations which propagate supersonically with detonation velocities in the range of km/s.
Pyrotechnic deflagrations
Combustion, or burning, is a high-temperature exothermic redox chemical reaction between a fuel and an oxidant, usually atmospheric oxygen, that produces oxidized, often gaseous products, in a mixture termed as smoke. Combustion does not always result in fire, because a flame is only visible when substances undergoing combustion vaporize, but when it does, a flame is a characteristic indicator of the reaction. While activation energy must be supplied to initiate combustion, the heat from a flame may provide enough energy to make the reaction self-sustaining. The study of combustion is known as combustion science.
The flames caused as a result of a fuel undergoing combustion (burning)
Air pollution abatement equipment provides combustion control for industrial processes.
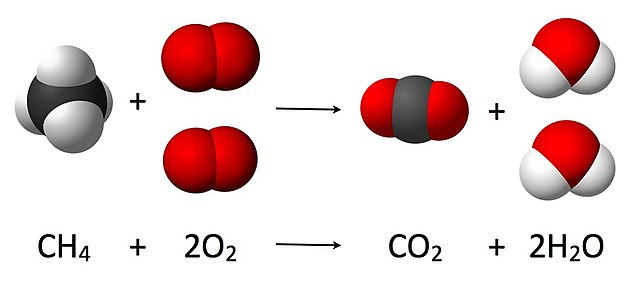
The combustion of methane, a hydrocarbon
Colourized gray-scale composite image of the individual frames from a video of a backlit fuel droplet burning in microgravity