A flame test is relatively quick test for the presence of some elements in a sample. The technique is archaic and of questionable reliability, but once was a component of qualitative inorganic analysis. The phenomenon is related to pyrotechnics and atomic emission spectroscopy. The color of the flames is understood through the principles of atomic electron transition and photoemission, where varying elements require distinct energy levels (photons) for electron transitions.
The flame test carried out on a copper halide. The characteristic bluish-green color of the flame is due to the copper.
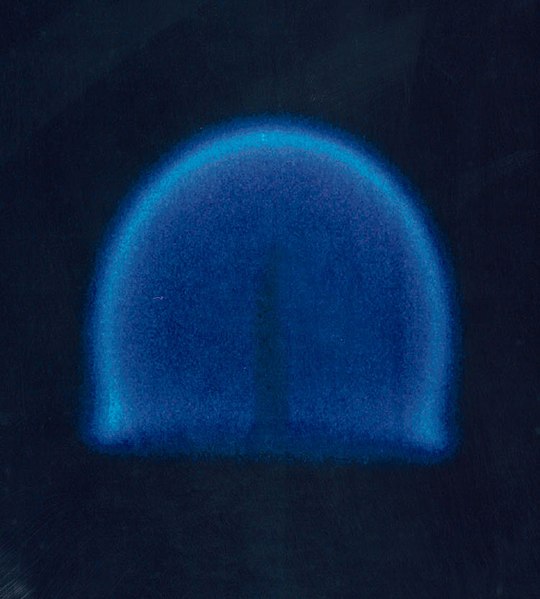
A flame test showing the presence of lithium.
Coloured flames of methanol solutions of different compounds, burning on cotton wool. From left to right: lithium chloride, strontium chloride, calcium chloride, sodium chloride, barium chloride, trimethyl borate, copper chloride, cesium chloride and potassium chloride.
Image: Zinc burning
A flame is the visible, gaseous part of a fire. It is caused by a highly exothermic chemical reaction taking place in a thin zone. When flames are hot enough to have ionized gaseous components of sufficient density, they are then considered plasma.
Flames of charcoal
Different flame types of a Bunsen burner depend on oxygen supply. On the left a rich fuel with no premixed oxygen produces a yellow sooty diffusion flame; on the right a lean fully oxygen premixed flame produces no soot and the flame color is produced by molecular radicals, especially CH and C2 band emission.
A flame test for sodium. The yellow color in this gas flame does not arise from the black-body emission of soot particles (as the flame is clearly a blue premixed complete combustion flame) but instead comes from the spectral line emission of sodium atoms, specifically the very intense sodium D lines.
In zero-G, convection does not carry the hot combustion products away from the fuel source, resulting in a spherical flame front.