Green fluorescent protein
The green fluorescent protein (GFP) is a protein that exhibits green fluorescence when exposed to light in the blue to ultraviolet range. The label GFP traditionally refers to the protein first isolated from the jellyfish Aequorea victoria and is sometimes called avGFP. However, GFPs have been found in other organisms including corals, sea anemones, zoanithids, copepods and lancelets.
Structure of the Aequorea victoria green fluorescent protein.
Aequorea victoria
The diversity of genetic mutations is illustrated by this San Diego beach scene drawn with living bacteria expressing 8 different colors of fluorescent proteins (derived from GFP and dsRed).
Different proteins produce different fluorescent colors when exposed to ultraviolet light.
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, responding to stimuli, providing structure to cells and organisms, and transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific 3D structure that determines its activity.
John Kendrew with model of myoglobin in progress
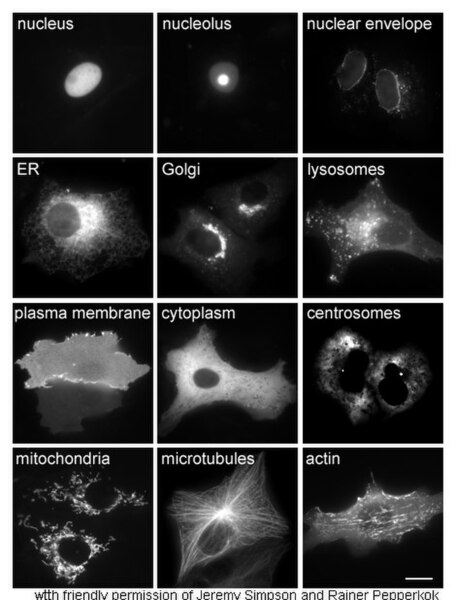
Proteins in different cellular compartments and structures tagged with green fluorescent protein (here, white)
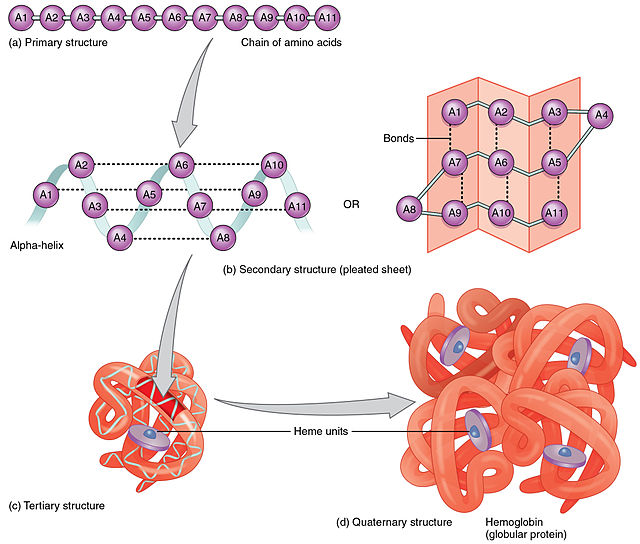
Constituent amino-acids can be analyzed to predict secondary, tertiary and quaternary protein structure, in this case hemoglobin containing heme units