Haptoglobin is the protein that in humans is encoded by the HP gene. In blood plasma, haptoglobin binds with high affinity to free hemoglobin released from erythrocytes, and thereby inhibits its deleterious oxidative activity. Compared to Hp, hemopexin binds to free heme. The haptoglobin-hemoglobin complex will then be removed by the reticuloendothelial system.
A model of α,β-hemoglobin/haptoglobin hexamer complex. There are 2 α,β-hemoglobin dimers depicted: one space filling model (yellow/orange), and one ribbon model (purple/blue). Each is bound by a haptoglobin molecule (both haptoglobin molecules are shown in pink, with one as a space filling model and one as a ribbon model).
Red blood cells (RBCs), scientific name erythrocytes (from ancient Greek erythros 'red' and kytos 'hollow vessel', with -cyte translated as 'cell' in modern usage), also known as red cells, erythroid cells, and rarely haematids, are the most common type of blood cell and the vertebrate's principal means of delivering oxygen (O2) to the body tissues—via blood flow through the circulatory system. Erythrocytes take up oxygen in the lungs, or in fish the gills, and release it into tissues while squeezing through the body's capillaries.
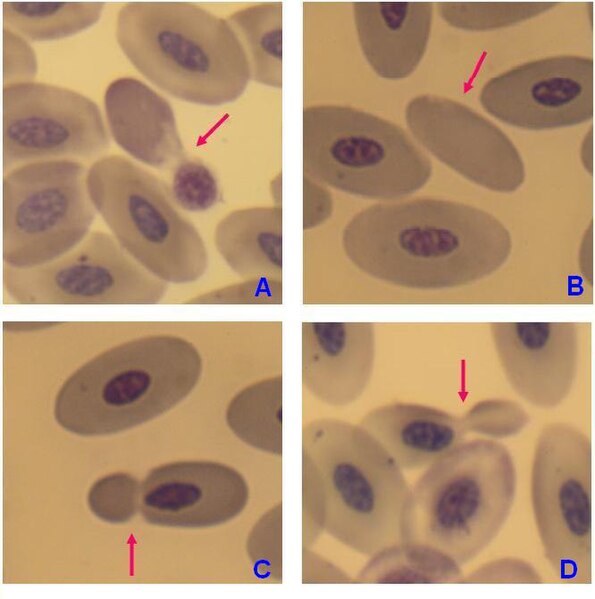
There is an immense size variation in vertebrate red blood cells, as well as a correlation between cell and nucleus size. Mammalian red blood cells, which do not contain nuclei, are considerably smaller than those of most other vertebrates.
Mature red blood cells of birds have a nucleus, however in the blood of adult females of penguin Pygoscelis papua enucleated red blood cells (B) have been observed, but with very low frequency.
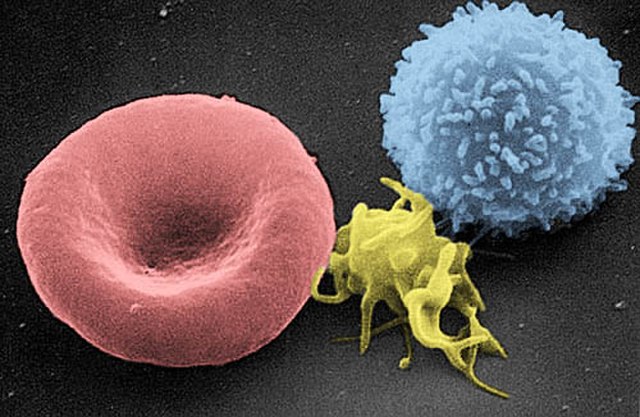
Scanning electron micrograph of blood cells. From left to right: human red blood cell, thrombocyte (platelet), leukocyte.
Two drops of blood are shown with a bright red oxygenated drop on the left and a darker red deoxygenated drop on the right.