In thermodynamics, heat is the thermal energy transferred between systems due to a temperature difference. In colloquial use, heat sometimes refers to thermal energy itself. Thermal energy is the kinetic energy of vibrating and colliding atoms in a substance.
A glowing-hot metal bar showing incandescence, the emission of light due to its temperature, is often recognized as a source of heat
Rudolf Clausius
A red-hot iron rod from which heat transfer to the surrounding environment will be primarily through radiation.
Joseph Black
Thermodynamics is a branch of physics that deals with heat, work, and temperature, and their relation to energy, entropy, and the physical properties of matter and radiation. The behavior of these quantities is governed by the four laws of thermodynamics, which convey a quantitative description using measurable macroscopic physical quantities, but may be explained in terms of microscopic constituents by statistical mechanics. Thermodynamics applies to a wide variety of topics in science and engineering, especially physical chemistry, biochemistry, chemical engineering and mechanical engineering, but also in other complex fields such as meteorology.
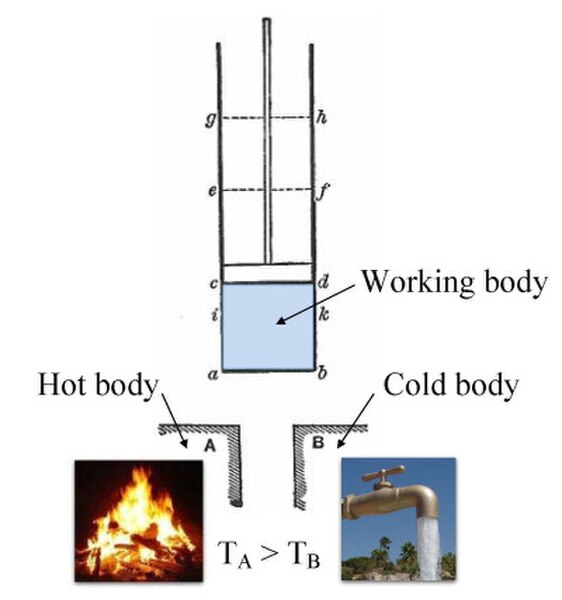
Annotated color version of the original 1824 Carnot heat engine showing the hot body (boiler), working body (system, steam), and cold body (water), the letters labeled according to the stopping points in Carnot cycle
Opening a bottle of sparkling wine (high-speed photography). The sudden drop of pressure causes a huge drop of temperature. The moisture in the air freezes, creating a smoke of tiny ice crystals.