High-performance liquid chromatography
High-performance liquid chromatography (HPLC), formerly referred to as high-pressure liquid chromatography, is a technique in analytical chemistry used to separate, identify, and quantify specific components in mixtures. The mixtures can originate from food, chemicals, pharmaceuticals, biological, environmental and agriculture, etc., which have been dissolved into liquid solutions.
A modern self-contained HPLC
At the ARS Natural Products Utilization Research Unit in Oxford, MS., a support scientist (r) extracts plant pigments that will be analyzed by a plant physiologist (l) using an HPLC system.
The quantitative parameters and equations which determine the extent of performance of the chromatographic system
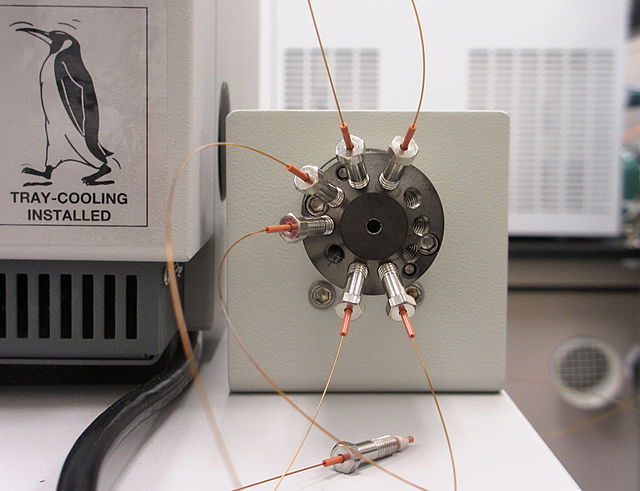
Tubing on a nano-liquid chromatography (nano-LC) system, used for very low flow capacities
In chemical analysis, chromatography is a laboratory technique for the separation of a mixture into its components. The mixture is dissolved in a fluid solvent called the mobile phase, which carries it through a system on which a material called the stationary phase is fixed. Because the different constituents of the mixture tend to have different affinities for the stationary phase and are retained for different lengths of time depending on their interactions with its surface sites, the constituents travel at different apparent velocities in the mobile fluid, causing them to separate. The separation is based on the differential partitioning between the mobile and the stationary phases. Subtle differences in a compound's partition coefficient result in differential retention on the stationary phase and thus affect the separation.
Thin-layer chromatography is used to separate components of a plant extract, illustrating the experiment with plant pigments which gave chromatography its name
Paper chromatography in progress
Thin layer chromatography
Two-dimensional chromatograph GCxGC-TOFMS at Chemical Faculty of GUT Gdańsk, Poland, 2016