Hsp90 is a chaperone protein that assists other proteins to fold properly, stabilizes proteins against heat stress, and aids in protein degradation. It also stabilizes a number of proteins required for tumor growth, which is why Hsp90 inhibitors are investigated as anti-cancer drugs.
Solid ribbon model of the yeast Hsp90-dimer (α-helices = red, β-sheets = cyan, loops = grey) in complex with ATP (red stick diagram).
Structure of the N-terminal domain of the yeast Hsp90 chaperone.
Crystallographic structure of the ATP binding pocket of Hsp90 where ATP is represented by a ball and stick figure (carbon atoms = grey, nitrogen = blue, oxygen = red, phosphorus = orange) and Hsp90 is depicted as a solid surface (negatively charged = red, positively charged = blue, electrostatically neutral = grey).
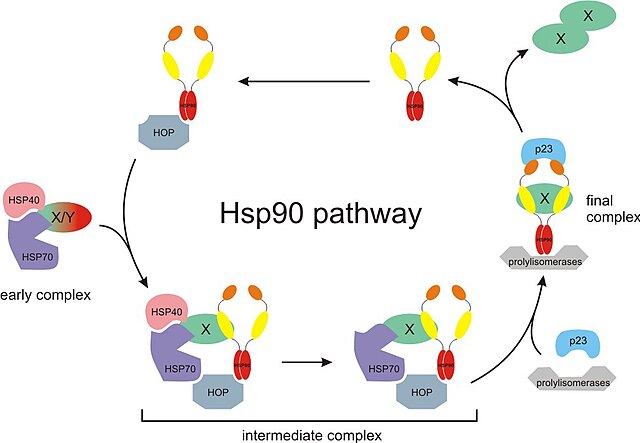
The Hsp90 chaperone cycle. X/Y represents an immature incompletely folded protein such a steroid receptor. Hsp40, Hsp70, and p23 are partner chaperones while Hop is a co-chaperone. Also, X-X represents a mature properly folded protein dimer.
Proteasomes are protein complexes which degrade unneeded or damaged proteins by proteolysis, a chemical reaction that breaks peptide bonds. Enzymes that help such reactions are called proteases.
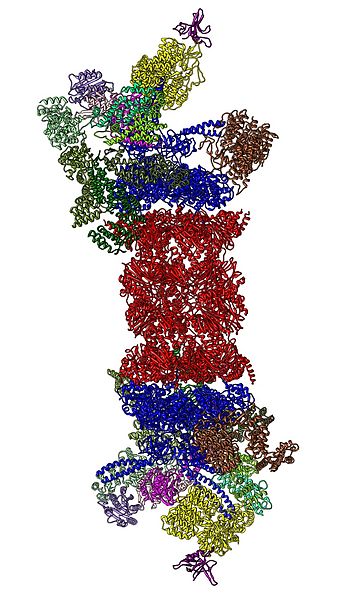
Cartoon representation of the 26S proteasome.