Julius Plücker was a German mathematician and physicist. He made fundamental contributions to the field of analytical geometry and was a pioneer in the investigations of cathode rays that led eventually to the discovery of the electron. He also vastly extended the study of Lamé curves.
Julius Plücker
Cathode rays or electron beams (e-beam) are streams of electrons observed in discharge tubes. If an evacuated glass tube is equipped with two electrodes and a voltage is applied, glass behind the positive electrode is observed to glow, due to electrons emitted from the cathode. They were first observed in 1859 by German physicist Julius Plücker and Johann Wilhelm Hittorf, and were named in 1876 by Eugen Goldstein Kathodenstrahlen, or cathode rays. In 1897, British physicist J. J. Thomson showed that cathode rays were composed of a previously unknown negatively charged particle, which was later named the electron. Cathode-ray tubes (CRTs) use a focused beam of electrons deflected by electric or magnetic fields to render an image on a screen.

A beam of cathode rays in a vacuum tube bent into a circle by a magnetic field generated by a Helmholtz coil. Cathode rays are normally invisible; in this demonstration Teltron tube, enough gas has been left in the tube for the gas atoms to luminesce when struck by the fast-moving electrons.
Crookes tube. The cathode (negative terminal) is on the right. The anode (positive terminal) is in the base of the tube at bottom.
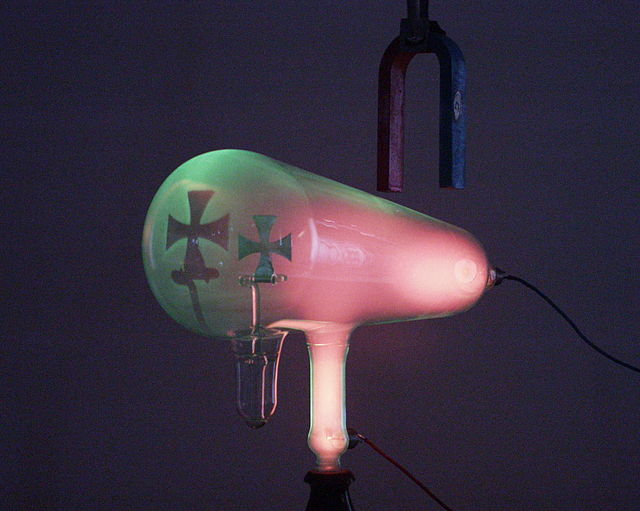
Cathode rays travel from the cathode at the rear of the tube, striking the glass front, making it glow green by fluorescence. A metal cross in the tube casts a shadow, demonstrating that the rays travel in straight lines.
A magnet creates a horizontal magnetic field through the neck of the tube, bending the rays up, so the shadow of the cross is higher.